Magnesium – Effects on Brazeability
For added strength and machineability, certain alloys contain Mg. Most notably are the 6XXX series alloys (up to 1% Mg) that are used for fittings and machined components and the so-called long life brazing sheet alloys (up to 0.3% Mg in the core). There is a limit to the amount of Mg tolerated in NOCOLOK® Flux brazing. Up to 0.5% Mg can be tolerated in furnace brazing while around 1% Mg is tolerable for flame brazing.
When an Al alloy containing Mg is heated, the Mg diffuses to the surface and reacts with the surface oxide to form MgO and spinels of MgO:Al2O3. The diffusion is time-temperature dependent and is rapid above 425°C. These spinel oxides have reduced solubility in the molten flux. Furthermore, Mg and/or MgO can react with the flux forming compounds such as MgF2, KMgF3 and K2MgF4. All of these serve to poison the flux and significantly reduce its effectiveness.
In flame brazing, higher Mg concentrations can be tolerated since the faster heating rates do not allow the diffusing Mg enough time to appreciably decrease the beneficial effects of the flux. Flame brazing components containing > 1% Mg may be possible under some circumstances with increased flux loadings and very fast heating rates (<20 second braze cycle).
It should be noted that when one speaks of the brazing tolerance to Mg, it is the total sum of the Mg concentrations in both components:
[Mg] component 1 + [Mg] component 2 = [Mg] total
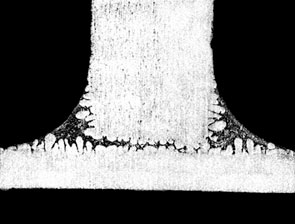
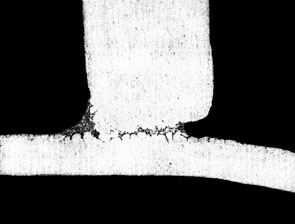
The figure below shows the effect of Mg on fillet size and geometry:
If the user is experiencing difficulties brazing and suspects elevated Mg levels as the cause, there are a couple of ways to be sure. First, check with the supplier of the alloys or perform a chemical composition analysis on the suspect alloys. This is the most certain way. Secondly, look for a golden hue on the brazed product. This is an indication that Mg alloys are being used and the color is a result of the increased oxide thickness. Furthermore, there may be a very light, almost fluffy residue on the brazed component that can literally be blown off by mouth. These visual indicators can most certainly be traced back to poor brazing results due to the presence of Mg.
Improving brazeability
There are a few ways in which the brazeability of Mg containing alloys can be improved:
- Increasing the flux loading. A substantial improvement is gained when increasing the flux loading up to 10 g/m2 or more in furnace brazing. In cases where there is just one component containing Mg such as in a fitting, extra flux can be brushed around the area of the joint.
- Increasing the heating rate. Slow heating rates allow more Mg to diffuse to the surface thereby hindering brazeability. For furnace brazing Mg containing alloys, the fastest possible heating rates achievable without sacrificing temperature uniformity will increase the tolerance to Mg.
- Combining increased flux loadings and faster heating rates.
- Maintaining proper gap tolerances and joint designs.
- Increasing the nitrogen flow rate to minimize furnace atmosphere contaminants that also compete to reduce brazeability.
Tip: NOCOLOK® Cs Flux
Better results are reported when using cesium containing fluxes for aluminum alloys containing Mg up to 0.6 – 0.8% Mg. Fewer leaks are observed when compared with standard flux and less porosity is noted in the joint areas. Furthermore, standard flux loads and braze cycles can be used with Cs containing fluxes.
NOCOLOK® Cs Flux is a flux of the general formula KxCsyAlFz where Cs is chemically bound. It has a melting range of 558°C – 566°C. The maximum Cs content is limited to 2% to keep the cost of the flux down. Increasing the Cs content does not increase brazeability as shown below:
Cesium reacts as a chemical buffer for Mg by forming CsMgF3 and/or Cs4Mg3F10. The flux inhibiting factors of Mg are therefore reduced.

Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!