Brazing of Aluminium Alloys withHigher Magnesium Content using Non-Corrosive Fluxes – Part 2
Technical Information by Leszek Orman, Hans-Walter Swidersky and Daniel Lauzon
Abstract
For just as long as aluminium has been used for brazing heat exchangers, there has been a trend to down-gauging components for weight savings. The most common alloying element to achieve higher strength alloys for the purpose of down-gauging is magnesium. While magnesium additions are helpful in achieving stronger alloys, the consequence is a decrease in brazeability. This article discusses the mechanism of brazing deterioration with the addition of magnesium and proposes the use of caesium compounds as a way of combating these effects.
We split the article in five parts:
- Introduction
- Effects of Mg on the Brazing Process
- Mechanism of Magnesium Interaction with the Brazing Process
- Caesium Fluoroaluminates
- NOCOLOK® Cs Flux
Effects of Mg on the Brazing Process
To illustrate the effects of Mg on the brazing process, Bolingbroke et al [4] chose the angle-on-coupon method. In this technique, an aluminium angle is laid on top of a cladded aluminium coupon where the legs of the angle are raised using stainless steel wire (see Fig. 1). Brazeability is thus measured as a function of the length of the fillet formed. In this set of experiments, the coupon base alloy is 3003 with Mg additions ranging from 0.1 to 0.58 w%. Only the coupon was fluxed at pre-defined loads ranging from 2 to 10 g/m2. The results of the Mg content on brazeability are shown in Fig. 2.
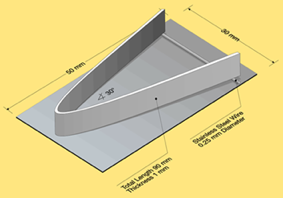
Fig. 1: Experimental set up for brazeability measurement [4].
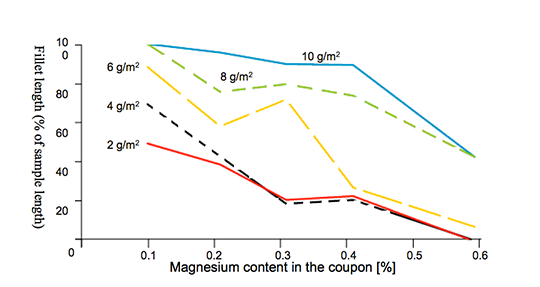
Fig. 2: Brazeability as a function of magnesium content [4].
Fig. 2 shows that increasing the flux load can reduce the negative influence of magnesium.
The solid state diffusion is time-temperature dependent and becomes rapid above 425°C. Thus brazing at higher heating rates should reduce the negative influence of Mg. The influence of heating rate on brazeability is shown in Fig. 3.
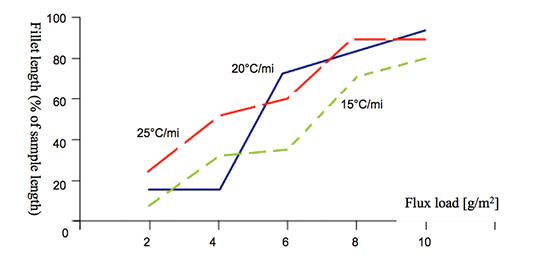
Fig. 3: Brazeability of 3003 alloy + 0.31 wt% Mg as a function of heating rate and flux load [4].
The influence of heating rates when kept within the values attainable for the CAB process is rather weak. Increasing the flux load is more effective in combating the negative influence of Mg for CAB processes.
In flame or induction brazing, where the heating rates are about two orders of magnitude higher than in the CAB process, alloys with Mg concentration even as high as 2% can be successfully brazed.
It should be noted that when one speaks of the brazing tolerance to Mg, it is always the total sum of the Mg concentrations in both components:
[Mg] component 1 + [Mg] component 2 = [Mg] total (1)
The effect of magnesium content on the appearance of the brazed joint is shown in Fig. 4.
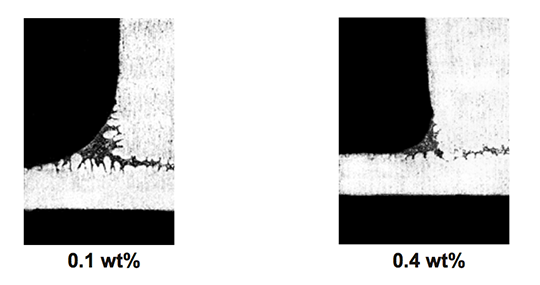
Fig. 4: Effect of Mg content on appearance of brazed joint [4].
At 0.1 wt% in the base coupon, the fillet is large and joining is complete. At 0.4 wt% Mg in the base coupon, the fillet volume is smaller.
Download the complete article as a PDF-File.
References:
- S. W. Haller, “A new Generation of Heat Exchanger Materials and Products”, 6th International Congress “Aluminum Brazing” Düsseldorf, Germany 2010
- R. Woods, “CAB Brazing Metallurgy”, 12th Annual International Invitational Aluminum Brazing Seminar, AFC Holcroft, NOVI, Michigan U.S.A. 2007
- T. Stenqvist, K. Lewin, R. Woods “A New Heat-treatable Fin Alloy for Use with Cs-bearing CAB flux” 7th Annual International Invitational Aluminum Brazing Seminar, AFC Holcroft, NOVI, Michigan U.S.A. 2002
- R. K. Bolingbroke, A. Gray, D. Lauzon, “Optimisation of Nocolok Brazing Conditions for Higher Strength Brazing Sheet”, SAE Technical Paper 971861, 1997
- M. Yamaguchi, H. Kawase and H. Koyama, ‘‘Brazeability of Al-Mg Alloys in Non Corrosive Flux Brazing’’, Furukawa review, No. 12, p. 139 – 144 (1993).
- A. Gray, A. Afseth, 2nd International Congress Aluminium Brazing, Düsseldorf, 2002
- H. Johansson, T. Stenqvist, H. Swidersky “Controlled Atmosphere Brazing of Heat Treatable Alloys with Cs Flux” VTMS6, Conference Proceedings, 2002
- U. Seseke-Koyro ‘‘New Developments in Non-corrosive Fluxes for Innovative Brazing’’, First International Congress Aluminium Brazing, Düsseldorf, Germany, 2000
- K. Suzuki, F. Miura, F. Shimizu; United States Patent; Patent Number: 4,689,092; Date of Patent: Aug. 25, 1987
- L. Orman, “Basic Metallurgy for Aluminum Brazing”, Materials for EABS & Solvay Fluor GmbH 11th Technical Training Seminar – The Theory and Practice of the Furnace and Flame Brazing of Aluminium, Hannover, 2012
- K. Suzuki, F. Miura, F. Shimizu; United States Patent; Patent Number: 4,670,067; Date of Patent: Jun. 2, 1987
- J. Garcia, C. Massoulier, and P. Faille, „Brazeability of Aluminum Alloys Containing Magnesium by CAB Process Using Cesium Flux,“ SAE Technical Paper 2001-01-1763, 2001

Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!