Summary
The article was written on the basis of frequently asked questions from companies which either wanted to start a new all-aluminium brazing production of heat exchangers or wanted to convert from copper and aluminium mechanical assembly design to all-aluminium brazed parts. The questions were grouped into three main categories: Equipment (emphasis on assembling process), Process (emphasis on different fluxes and fluxing methods) and Corrosion.
Specific production challenges are also presented, which are important not only to newcomers of all-aluminium brazed heat exchangers, but to established companies as well. These include typical brazing problems such as managing leaks and the basics of brazing copper to aluminium. These topics are discussed by their relevance to the brazing parameters and their role in successful brazing.
Content:
- Introduction (Part 1, issue July 2013)
- Equipment (Part 1, issue July 2013)
- Brazing process (Part 2, issue August 2013)
- Brazing copper to aluminium (in this issue)
- Corrosion resistance (in this issue)
- Summary (in this issue)
4. Brazing copper to aluminium
When replacing a heat exchanger in an existing design, very often the connecting pipes are made from copper. Therefore the typical question: ”Is it possible to braze aluminium pipe to a copper one?” The answer is: Yes, it is possible by flame brazing. At 548°C there is the formation of a eutectic between copper and aluminium. This reaction is very rapid; therefore accurate temperature control and short process times such as with flame brazing are required. It is easier to braze at a temperature below the eutectic formation, thus lower melting point filler alloy and flux are required. In this case the recommended filler alloy would be ZnAl and Cs-Al-F flux. When copper remains in contact with aluminium for a longer period of time, such as in furnace brazing, an intensive dissolution of aluminium is observed. Therefore, for any factory which has production of copper and aluminium brazed exchangers, it is of very high importance to keep those two activities well separated from each other. A result of contamination of a condenser tube with a small chip of copper is shown in fig 7.

Fig. 7: Hole in a brazed tube surface burned through by a copper chip
When joining copper to aluminium it must be remembered that extreme galvanic corrosion can take place when the joint is exposed to a humid or wet environment. It is therefore obligatory to make sure that Cu-Al joints are not exposed to water during service. This can be achieved for example by using temperature shrinking plastic sleeves over the tube joint.
5. Corrosion resistance
Corrosion resistance of condensers for air conditioning system is one of the major utility properties. Thus the first question: ”Is there any approved test for determining the corrosion resistance requirements for HVAC heat exchangers?” Unfortunately the HVAC industry has not yet developed a commonly accepted test standard for assessing corrosion resistance. In the automotive world, the most common tests used by manufacturers are:
- SWAAT (ASTM G85 annex A3) – seawater acidified test, cyclic; it is an aggressive corrosion test commonly used in the automotive industry, but the characteristic of the test does not correspond well to the working conditions of stationary units.
- Salt Spray Test (ASTM-B-117, ISO 9227), it is a test better reflecting the working conditions of stationary units, but it is not sufficiently aggressive (too long time for completion).
Other methods developed in response to observed corrosion due to rain or condensation water remaining on the units for a prolonged time, is the socalled soaking or water-exposure test. In this experiment a small cut-out heat exchanger section is immersed in demineralized water for a certain period of time and the concentration of ions in the water after soaking is analyzed. The procedure has not been standardized, therefore it is not really possible to compare results obtained by different companies, but the test can be used for direct comparison of different fluxes and materials. For now no correlation between its results and real life time has been established.
Invariably many companies when considering production of brazed aluminium heat exchange ask a question: ”What sort of alloys should be chosen for the best corrosion performance?” This topic is quite complex and there is no single “best answer”. In the authors’ opinion the best method is to discuss the subject with the aluminium suppliers who have a lot of knowledge and experience in choosing the optimal aluminium alloys. Every heat exchanger is an assembly of different components and when considering its corrosion resistance, the alloys of individual elements should be looked at as a unit in which mutual interactions between each component are taking place.
There are many different working environments which will significantly influence the corrosion behaviour of the parts. According to [8] the following major types can be distinguished:
Coastal/Marine:
This environment is characterized by an abundance of sodium chloride and sulphur compounds carried by spray, fog or winds.
Industrial:
This environment can be much diversified, where sulphur and nitrogen contaminants are most notable.. Many of the gases emitted during different combustion processes come back to the ground in form of acid rain. Also this environment produces a lot of different small particles in the form of dust which covers the equipment creating potential increased corrosion hazards.
Combination Marine/Industrial:
A combination of the above two factor create the harshest environment for any HVAC equipment.
Urban:
This environment is characterized by high level of automobile and house heating emissions. These are mainly SO2 and NOx compounds resulting also in acid rains.
Rural:
Usually these are unpolluted areas; however in some cases pollution may appear with higher a concentration of ammonia and nitrogen originating from animal excrement and fertilizer use.
The best solution would be to choose the alloys according to the different working environments; this however has hardly ever been possible.
As a mater of fact, [8] suggests that in particularly aggressive environment the coils should always have additional protection layer/coating.
6. Summary
Thanks to technical advantages of brazed heat exchangers over the mechanical ones and driven by high copper prices, it seems that a change into all aluminium heat exchangers in the HVAC&R industry is inevitable. Though the process of conversion from copper and /or mechanically assembled heat exchangers is in most cases a significant challenge, when properly planned it can be done smoothly without any unpredictable surprises. The major aspects which should be considered are equipment choices with a special emphasis on the assembly method, selection of proper alloys and the most optimal fluxing technology. The required data for the project and investment decisions can be obtained by direct contacts with equipment and consumables manufacturers.
References:
8. Selection Guide: Environmental Corrosion Protec-tion, Carrier Corporation, Syracuse, New York, July 2009
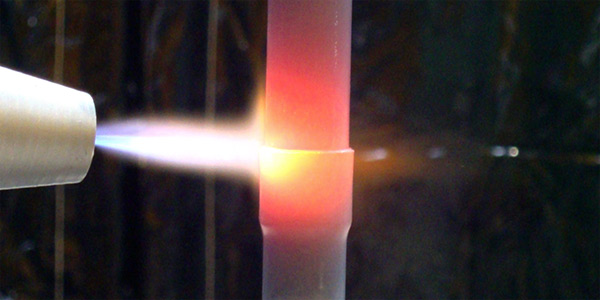




 flame brazing of aluminium to copper.
flame brazing of aluminium to copper.