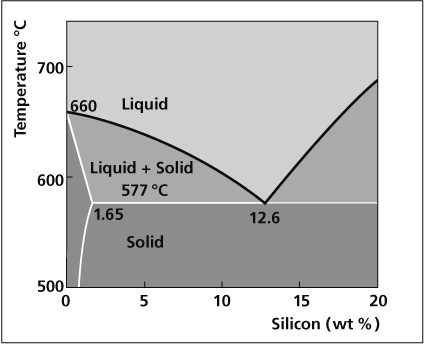
Dust and dirt, condensates, lubricants and oils must be thoroughly removed. If the metal work pieces are poorly prepared, the flux will not spread evenly and the flow of filler alloy will be haphazard: it will either not spread properly or will discolour. The consequence would be an incomplete joint.
The first step is therefore: always clean the components of all oil and grease. The surfaces can be cleaned using either chemical, water-based or thermal cleaning techniques and substances.
Aqueous Cleaning
Aqueous or water based cleaning is a quite efficient and robust process, but still generates some waste water.
Aqueous cleaning starts off with a concentrated metal cleaning agent, which is subsequently diluted with water to 1% to 5% (v/v). The composition of a supplier’s cleaning solution is proprietary, but usually contains a mixture of surfactants, detergents and active ingredients such as sodium carbonate that serves to elevate the pH. Once diluted, the cleaning solution will typically have an elevated pH in the range of pH 9 to 12. There are acid based solutions, but appear to be less common.
The best water-based cleaners contain water, tensides, cleaning agent and active ingredients such as carbonates.
The cleaning solution works best at higher temperatures and is usually recommended to operate at 50°C to 80°C. Cleaning action is quicker at higher solution temperatures.
Thermal Degreasing
Thermal degreasing works by elevating the temperature of the work piece so that lubricants present on the surfaces will be evaporated. This procedure only works with special types of lubricants known as evaporative or vanishing oils. Vanishing oils are light duty lubricants used mostly for the fabrication of heat exchanger fins, although they are now finding uses in the stamping and forming of other heat exchanger components. Lubricants not designed for thermal degreasing must not be used. These could leave behind thermal decomposition products and carbonaceous residues which at higher level prevent brazing and have the potential to degrade product appearance and accelerate corrosion.