One of the largest potentials to increase efficiency of heat exchangers lies within the heat-transfers: reducing condensing temperatures by 3 °K will improve overall system efficiency by approx. 10 % for a standard R 410 A air conditioning cycle. A minimization of the temperature difference between the air flows and the phase change temperatures of the refrigerants can be achieved by improving the heat transfer efficiency of the heat exchangers. Brazed microchannel heat exchangers have already proven that they are a cost effective solution for the utilization of this optimization potential – as well as boasting a number of other benefits (see below). Brazed microchannel heat exchangers have been the technology of choice in the automotive industry for the past 10 to 15 years, and are already making inroads into the stationary HVAC&R industry for the following convincing reasons.
Poor contacts between fins and tubes account for approximately 5 – 10 % of heat transfer resistance in a standard heat exchanger manufactured by mechanical or hydraulic expansion of the round tubes because this always leaves imperfect connections between the parts. The microscopic image shows the small gaps between fins and tubes responsible for contact resistance that reduces heat transfer performance.

Fig. 1: Small gaps between fins and tubes reduce heat transfer performance in mechanically or hydraulically manufactured heat exchangers.
Brazed connections are much better because they metallurgically bond the fins and tubes in a single conductive material, eliminating all potential sources of contact resistance.
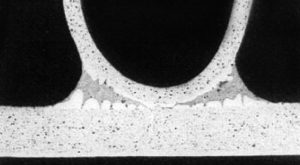
Fig. 2 Excellant heat transfer performance because no gaps in brazed connections.
