In everyday practice we can encounter problems connected with brazing imperfections – often manifested as leaking. In order to identify the failure root causes, usually a detailed examination needs to be perform. Optical metallography is one of the most important tools for such an analysis.
Schlagwortarchiv für: Silicon
In everyday practice we can encounter problems connected with brazing imperfections – often manifested as leaking. In order to identify the failure root causes, usually a detailed examination needs to be perform. Optical metallography is one of the most important tools for such an analysis.
Process related causes
The service life of a heat exchanger may be shortened due to corrosion caused by process related events. Some examples are listed below:
Excessively high brazing temperature or too long time at temperature will lead to excessive Si diffusion in the core. Si diffuses along grain boundaries and this can increase the susceptibility to intergranular corrosion. By maintaining proper time-temperature cycles and thereby minimizing Si diffusion, intergranular attack can also be minimized.
Copper in contact with aluminum will cause a corrosion related failure very quickly. Copper is noble (cathodic) to aluminum and when these two metals are in contact in the presence of an electrolyte, the aluminum will be consumed rapidly. This may occur in a heat exchanger manufacturing facility where both Al and Cu heat exchangers are produced and there is cross-contamination of process routes. It only takes one small Cu chip to land on the surface of Al during some part of the manufacturing process to cause a short-term failure in the Al heat exchanger. If both Al and Cu heat exchangers are to manufactured under the same roof, it is recommended (and practiced) to physically separate the two production routes with a wall and take extensive steps to avoid cross-contamination.
Carbonaceous residues can be generated on the heat exchanger surfaces during the heat cycle from residual lubricants, excessive use of surfactants, binders in flux or braze pastes etc. Carbon plays very much the same role as Cu in that it is noble to Al. In a corrosive environment, carbon residues act as a cathode and Al as an anode, leading to the galvanic corrosion of Al. The best preventative measure is to ensure that the heat exchangers are thoroughly and properly cleaned and degreased prior to brazing. This includes monitoring the flux slurry bath for any signs of organic contamination (for instance oil slicks).
Coatings
Painting a heat exchanger offers some level of corrosion protection, but is primarily used for cosmetic purposes. Painting will enhance corrosion protection if it covers the entire heat exchanger uniformly and is free from defects. In fact, paint defects or stone chips will accelerate corrosion locally. Many Al producers believe it is better to leave the heat exchanger unpainted to prolong its service life.
Conversion coatings such as chromate or phosphate conversion coatings work differently than painted surfaces. Conversion coatings enhance the natural oxide film on Al, essentially making it thicker and more resistant to hydrolysis. These types of coatings are most often used with automotive evaporators.
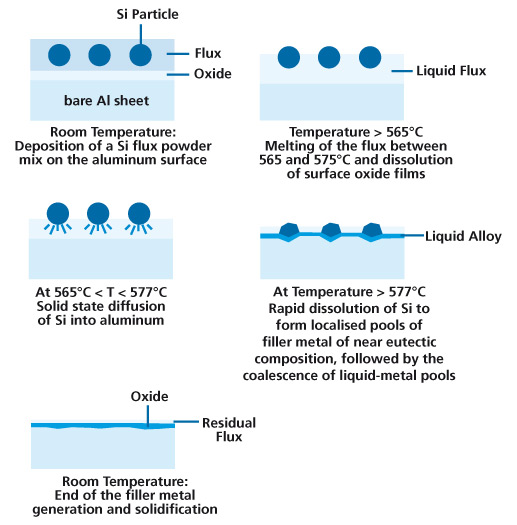
NOCOLOK® Sil Flux brazing is a technique, which eliminates the need for clad brazing sheet or conventional Al-Si filler metal. Sil flux brazing uses filler metal generated in-situ to effect brazing. The mechanism for creating this filler metal in-situ is described below:
- One of the surfaces to be joined is coated with a mixture of NOCOLOK® Flux and metallic Si powder. The coated assembly is then heated in the same fashion as in conventional furnace or flame brazing techniques.
- As the temperature rises, the flux melts at 565°C, dissolving the oxides on both the Al substrates and the Si particles.
- The bare Al surface is now in contact with metallic Si, and in the absence of oxides, allows solid-state inter-diffusion of Al and Si. Very quickly the composition near a Si particle reaches that of the Al-Si eutectic (Al-12.6% Si).
- As the temperature increases beyond the eutectic reaction temperature of 577°C, the formation of a liquid pool is established. The formation of the liquid leads to rapid dissolution of the remaining Si through liquid diffusion. The pool of liquid continues to grow, consuming Al, until all of the Si is consumed in the melt. In the presence of a joint, the liquid pool is drawn to the joint by capillary action.
- Upon cooling, the liquid layer solidifies to form a metallurgical bond between the components.
Sil Flux Process
When brazing aluminum to stainless steel using:
a) NOCOLOK® Flux and Al-Si filler alloys are suitable
or
b) alternatively CsAlF-Complex flux (melting range between 420 and 480°C) and Zn-Al filler alloys.
Regarding a): Brazing of aluminum to stainless steel works both with NOCOLOK® Flux + Al-Si filler alloy and with NOCOLOK® Sil Flux. After the flux melts and the oxides are removed, there is a reaction between Al and Fe, forming a thin intermetallic layer of FeAl3. This layer forms the metallurgical bond between the Fe and Al components. FeAl3 is very brittle and thus the thickness of this layer should be minimized, otherwise the joint can easily fracture.
From a metallurgraphic point of view, there is a multi-layer system (microscopic structures). First, there is the stainless steel, then the layer of FeAl3, then the Al/Si filler metal, and finally the aluminum base material. The thickness of the brittle FeAl3 layer is a function of brazing time and temperature; – consequently the need for a short brazing cycle with fast heat-up and very short holding time at maximum temperature. Too high brazing temperatures must be strictly avoided. Only with a short brazing cycle, successful joining of aluminum to steel is possible.
Joining of Al to steel using NOCOLOK® Flux is done on large scale commercially for the production of pots and pans (stainless steel pots with aluminum ‚compensation base plates‘) – mostly in induction brazing. It is also used for the production of heating elements (steel heating plates with aluminum base plates and tubes for the electrical heating wires). Another application for aluminum to steel joining is brazing of large aluminum-plated steel tubes – up to 11 meters long – with aluminum fins for power plant cooling modules.
In the manufacturing of pots and pans where there is a large surface area between the Al base plate and the pot, a mixture of filler metal powder and flux is often used. This circumvents the use of filler metal shim stock which is said to be costly and difficult to implement. In Al tube to steel or stainless steel tube joining, conventional flame brazing techniques can be used. Filler metal wire, either pre-placed or fed into the joint must be used. In the production of power plant cooling modules (with aluminum-plated steel tubes), the filler alloy is provided by clad fin material.
The following article provides some answers on general questions regarding the use of NOCOLOK Sil Flux for manufacturing pots and pans.
What is the NOCOLOK Sil Flux quantity (per m²) required for sandwich brazing or pressure cookers (stainless steel to aluminium)?
The recommended load for NOCOLOK Sil Flux is approximately 15 to 25 g/m². Brazing aluminium to stainless steel requires rapid processing, i.e. very fast heating ramp and short time at brazing temperature. Usually, this can only be accomplished with induction brazing.
When brazing aluminium to stainless steel using NOCOLOK Sil Flux, the Sil Flux first forms the filler metal from the aluminium component. The filler metal then reacts with the stainless steel to form a thin layer of FeAl3.
From a metallurgraphic point of view, there is a multi-layer system (microscopic structures). First, there is the stainless steel, then the layer of FeAl3, then the Al/Si filler metal, and finally the aluminium substrate. The FeAl3 layer is very brittle, and so it is important that this layer is kept as thin as possible. The thickness of this layer is a function of time and temperature,- consequently the need for a short brazing cycle.
To prepare a NOCOLOK Sil Flux slurry or paste: What is the exact mixing ratio (flux to solvent) required?
The mixing ratio for NOCOLOK Sil Flux slurries or pasts depends on the application method on site. In some cases, the main focus is a specific viscosity for an automated fluxing system. In other cases, only small flux quantities are prepared for immediate consumption.
NOCOLOK Sil Flux can be prepared with alcohol (ethanol or isopropyl alcohol) or alcohol/ water mixtures (70% alcohol content) in any ratio from 20 to 60 wt% (solids). As mentioned earlier, the actual slurry concentration will depend on the application procedure. The objective is to achieve 15 to 25 g/m² surface area.
If the NOCOLOK Sil Flux slurry is not completely consumed within one or two days, we recommend using pure alcohol as carrier to avoid any chemical reaction between the solvent and the metal powder (silicon). Due to hydrolysis of the silicon powder, water should not be used to prepare NOCOLOK Sil Flux paste.
Brushing, dipping or spraying can be utilised to apply the flux. Uniformity of the applied flux coating is very important.
How fast after applying NOCOLOK Sil Flux, the components should be processed for best results?
Before the part is heated up, the NOCOLOK Sil Flux slurry or past coating on the component surfaces should be thoroughly dried or allowed to evaporate. If alcohol is used as a carrier, the evaporation will only take a few seconds (with 15 to 25 g/m² flux load). NOCOLOK Sil Flux is non hygroscopic (i.e. the flux does not attract and absorb moisture) and non-corrosive (i.e. there is no reaction between the flux and the metal surfaces at room temperature). If a water mixture is used as the flux carrier, the components must be dried after flux application to avoid water-based corrosion effects.
What is the grain/ particle size distribution of the silicon metal powder in NOCOLOK Sil Flux?
The silicon particles in NOCOLOK Sil Flux show a particle size distribution curve with most of the grains within a range of 10 to 45μm. The Solvay specification for NOCOLOK Sil Flux (fine grade) – which is used for brazing pots and pans – is as follows:
< 5μm: < 25%
10 to 20μm: 50%
> 35μm: < 10%
> 74μm: not detectable (by laser particle size analysis)
What are the key points regarding product fit-up for good joint formation in NOCOLOK Sil Flux technology?
During the brazing process it is important that the components to be joined are in intermediate contact with each other. There must be firm pressure applied on all the surfaces of the plates throughout the brazing cycle to avoid large voids and gaps. Filler metal can only fill gaps up to a certain width (i.e. approximately 0.10 to 0.15 mm).

