Case Study
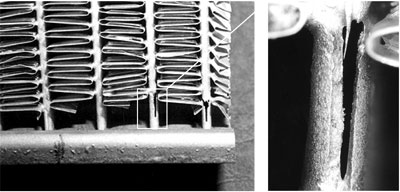
A radiator core retrieved from service was examined for a suspected premature corrosion related failure.
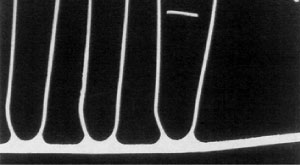
Upon closer metallographic examination, no evidence of corrosion was found at the failed area.
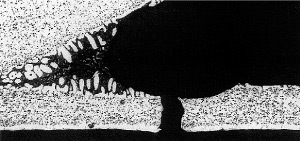
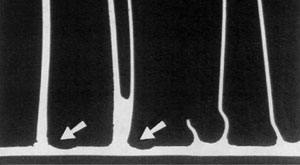
33% tube core erosion in the failure area
Header: AA4343/ AA3005
Tube: AA4343/ AA3003
It was concluded that the cause of the failure was in fact a mechanical failure occuring in the thinned wall area.
The following sequence of events proposes a rational explanation for the eroded tube area:
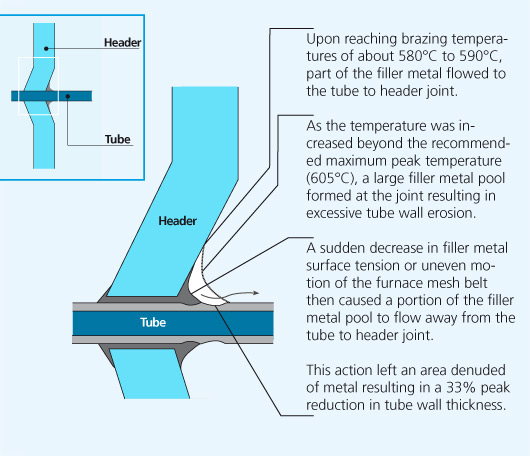
In service radiators are subject to internal pressure fluctuations and expansion and contraction due to heating and cooling. Mechanical failure was imminent and occured in the weakest part of the tube, the thinned down tube wall area adjacent to the tube to header joint.
Conclusions
Erosion of the base metal is undesirable since it reduces the wall thickness of the brazed component.
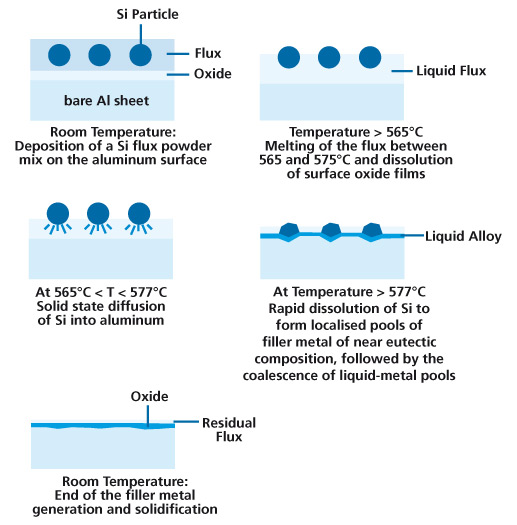
In addition Si penetration in the grain boundaries is known to increase the susceptibility to intergranular corrosion. Therefore proper filler metal management practices should be observed to prevent undesirable effects. One such factor easily controlled by the brazer is maximum peak brazing temperature.