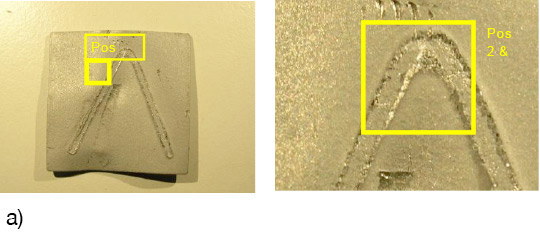
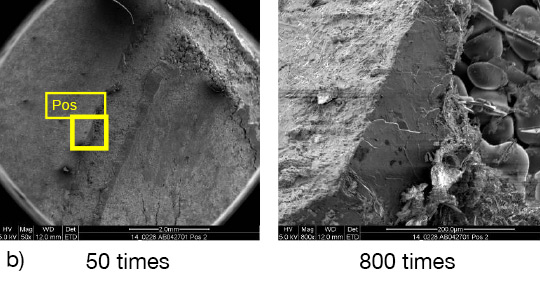
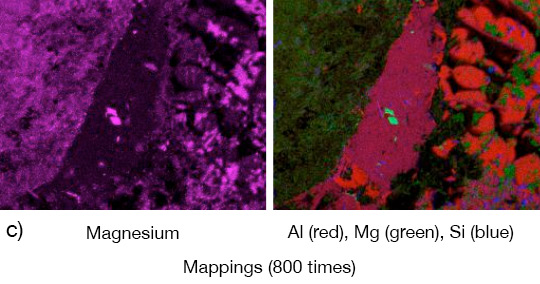
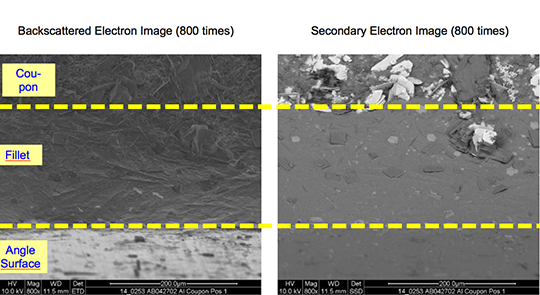
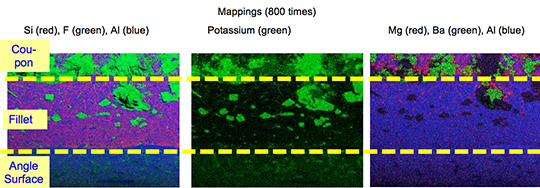
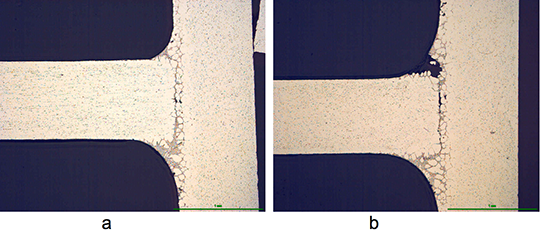
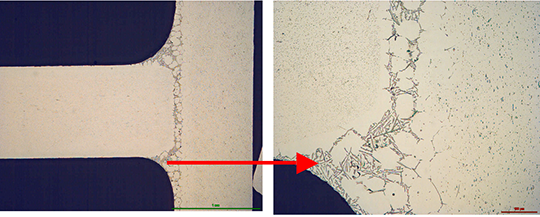
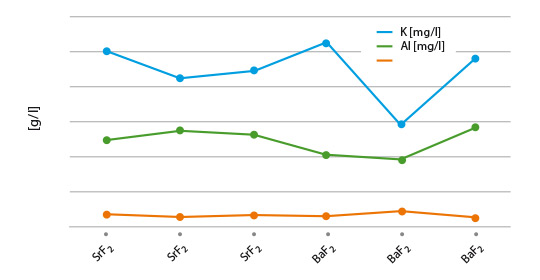
What is NOCOLOK® Cs Flux (SM)? (Synthesized Material)
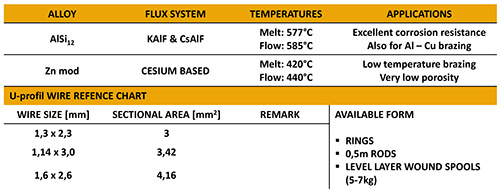
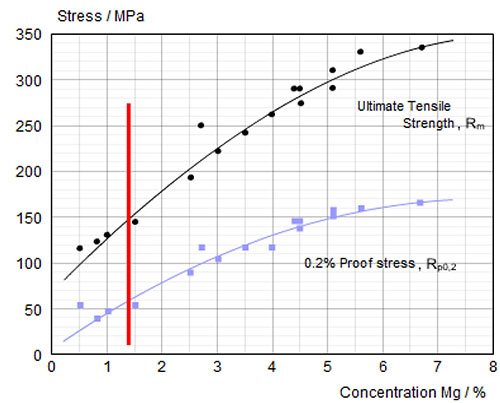
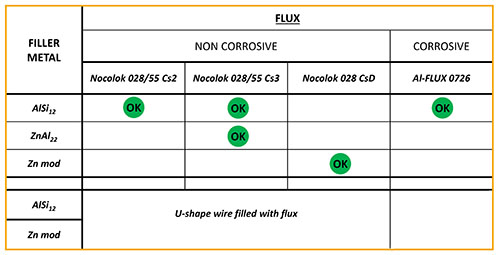
NOCOLOK® Cs Flux is used for brazing of aluminium alloys with higher magnesium levels. The Cs flux currently available for CAB (Controlled Atmosphere Brazing – furnace brazing) is a technical mixture (i.e. a mechanical blend) of K-Al-F flux (NOCOLOK® Flux) with Cs-Al-F flux – this product is offered under the name NOCOLOK® Cs Flux (TM): Technical Mixture.
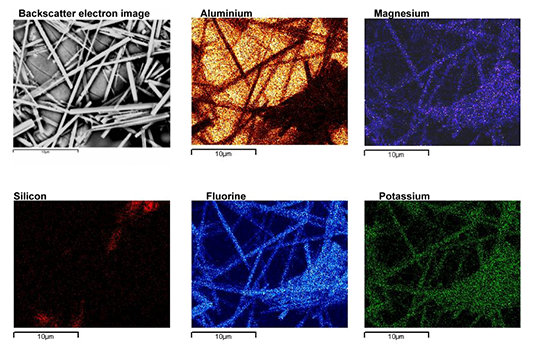
The new NOCOLOK® Cs Flux (SM) is a fully synthesized material – i.e. a unique and homogenous product. The Cs is completely embedded in a Cs-K-Al-F matrix during the manufacturing process.
Advantages
When comparing the characteristics and application of blended Cs Flux „(TM)“ with synthesized material „(SM)“, there are notable advantages of the new quality:
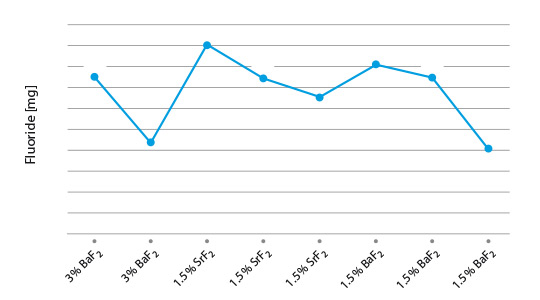
- The mixture can show settling and separation in flux slurries and paints. This is caused by differences in the density, the particle size, and the solubility of the two compounds in the blend.
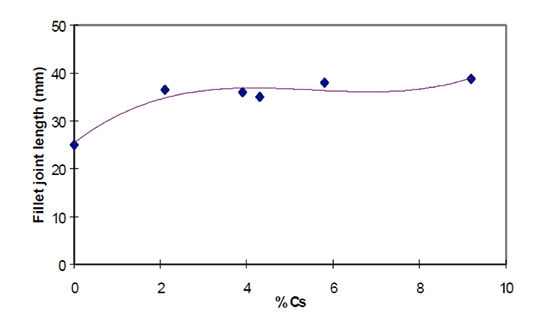
- In the new fully synthesized material – with the Cs completely incorporated in a Cs-K-Al-F matrix – the density is consistent and the particle size more uniform. We have a homogeneous powder with improved stability in suspensions (i.e. for slurries, paints, and pastes).
- In addition, the overall solubility is reduced when compared with the blended material.
- There will be less settling and less separation – which means that there is enhanced application performance with NOCOLOK® Cs Flux (SM).
NOCOLOK® Cs Flux (SM) is on stock at our Wimpfen facility and available right away.
Worldwide Registration
For a number of years, more and more countries are converting their existing chemical regulations or are implementing new regulations. In many cases, these regulations can be considered as an adaption of the European REACH Regulation. A registration of chemical substances or reaction masses is required, including comprehensive material data sets and risk assessment.
Solvay appreciates and supports these new product safety initiatives.
As a consequence, however, this leads to that in order to fulfill all regulatory requirements new products can only be introduced stepwise to other countries.
NOCOLOK® Cs Flux (SM) has already been successfully registered according to the European REACH Regulation and can be used without restriction within the European Union. Please also refer to our Safety Data Sheet, which is available on request. Registration for other countries/regions will be done successive. For more information, please contact our local sales offices.