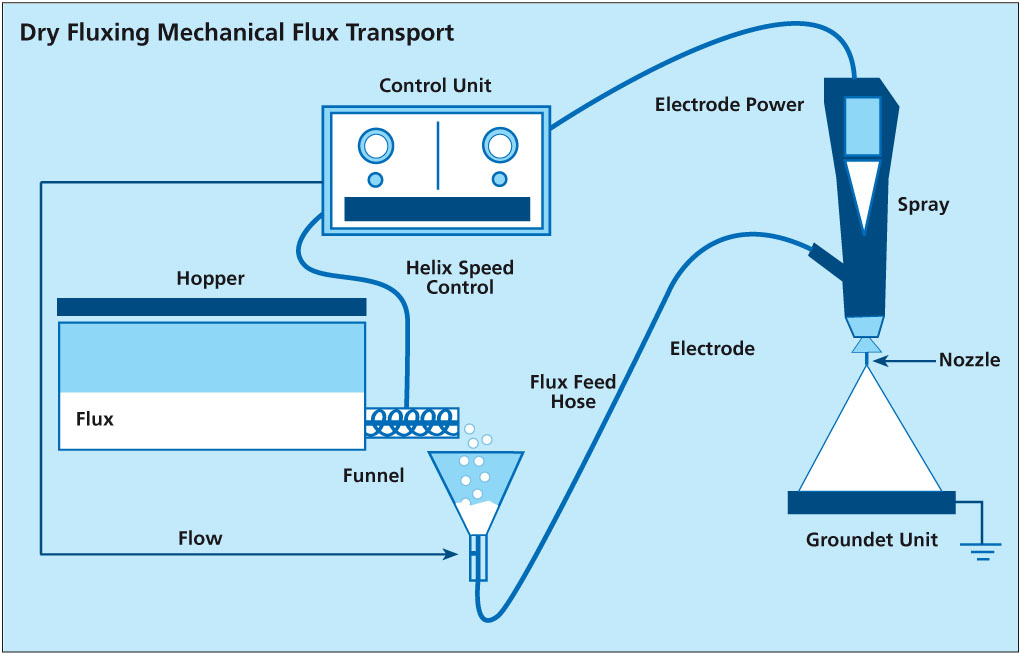
written by Leszek Orman, Radziszow / Poland; Hans-Walter Swidersky, Hannover / Germany
3 Results and discussion / Part 2
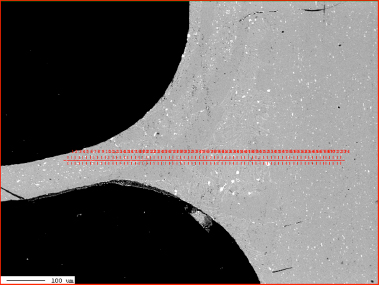
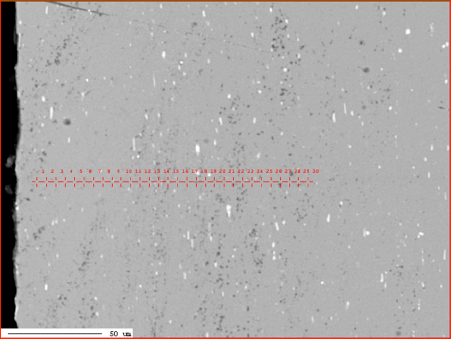
Corrosion resistance of the mock up samples was checked in the so called soaking test. Exemplary structures are shown in fig. 4:
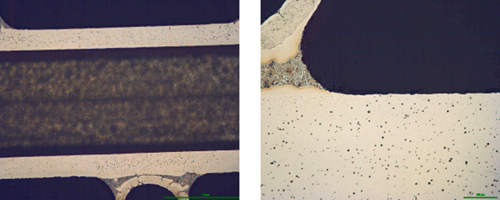
Fig. 4: Exemplary metallographic cross sections after 60 days of soaking (flux load 16g/m2, tube: HA 9170)
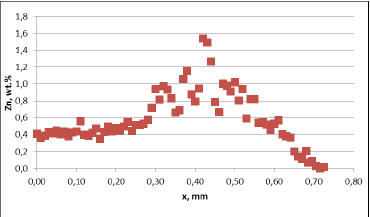
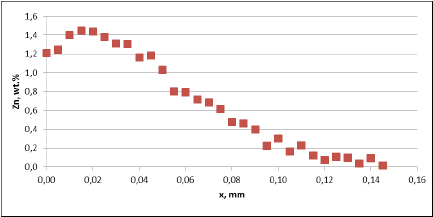
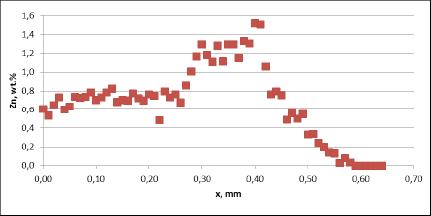
Observation of metallographic structures did not allow for a univocal statement that there are certain differences between investigated parts. In none of the investigated cases a fin debonding process was observed. At longer time of soaking, intensive corrosion of fins could be observed. More details were obtained when measuring the thickness of the tube walls. These results are presented in fig. 5:
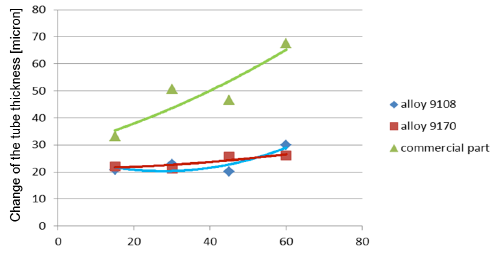
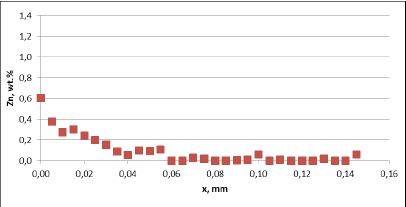
Fig. 5a: Change in the tube wall thickness as a function of soaking time – Time of soaking [days] – Flux load 8 g/m2
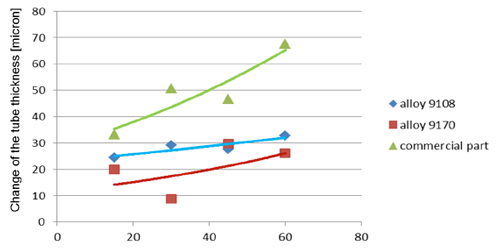
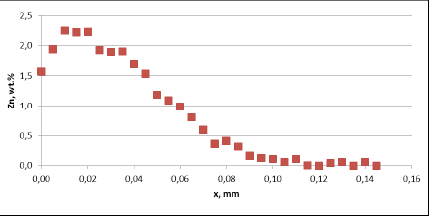
Fig. 5b: Change in the tube wall thickness as a function of soaking time – Time of soaking [days] – Flux load 16 g/m2
It seems that thinning of tubes at the beginning of the soaking test is quite rapid as compared to the period of soaking longer than 15 days. In case of the NOCOLOK® ZnLi Flux, the maximum tube thinning is in the range of 30 microns. The same maximum value for the commercial part is in the range of 65 microns. There is no significant difference in the tube thinning between the two tested different alloys.
Industrial test
The industrial test was performed with condensers used for stationary application. However, they had a typical design as for automotive. In this case – since the standard production tube were not zinc coated – the testing was done using NOCOLOK® Li Flux.
Material of the tested part:
Fins: FA 6815 clad 4343 both sides
Tubes: HA9108
Manifolds: HA3905-R outside clad HA4045-D
Brazing was performed on industrial brazing line made of wet fluxer, dryer and Active Only (Seco/Warwick) brazing furnace. Fluxing was done with NOCOLOK® Li Flux – concentration 13% for overhead spray and 43% for tube to manifolds joints. The above concentrations were the same as for standard industrial production. Also no changes were done in brazing parameters as compared to standard conditions.
Corrosion resistance of the brazed parts was checked by SWAAT (ASTM G85 A3). In order to examine the parts after different times at SWAAT, a brazed part was segmented, and all the segments were tested in the same test chamber – but for different time periods.
Examination of the segments after 5 days in SWAAT did not reveal any significant progress of corrosion; however, after longer times, fin debonding was observed. This examination did not show any differences between the parts fluxed with NOCOLOK® Li Flux and those fluxed with standard NOCOLOK® Flux. More information was obtained when examining metallographic cross sections:
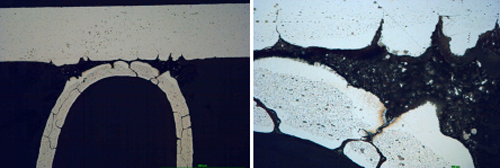
Fig. 6a: Structure of the samples brazed with NOCOLOK® Flux after 5 days in SWAAT
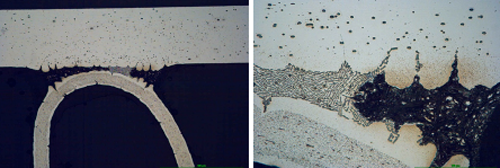
Fig. 6b: Structure of the samples brazed with NOCOLOK® Li Flux after 5 days in SWAAT
It can be observe that initially corrosion starts as intergranular attack on the fin material and as an intensive attack on the filler alloy. The filler is attacked selectively; firstly the alpha phase of the eutectic is removed.

Fig. 7: Structure of samples brazed with NOCOLOK® Flux (left) and NOCOLOK® Li Flux (right) flux after 60 days in SWAAT
Already after 30 days of SWAAT, for both sample almost all the fins were detached from the tubes – thus the examination of the parts after 60 days of SWAAT was limited only to the tubes.
The surface of the tube in case of standard NOCOLOK® is corroded to a much higher degree than in the case of the part brazed with NOCOLOK® Li Flux. The corrosion in both cases is initiated by intergranular attack; however later on it does not develop into pitting corrosion, but the removal of the tube material has laminar character. It seems that NOCOLOK® Li Flux did not cause a change of the corrosion mechanism; it just delayed the corrosion processes.
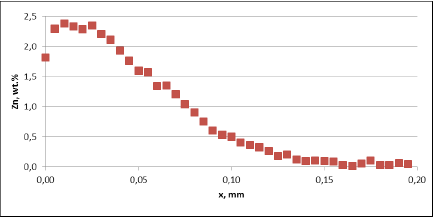
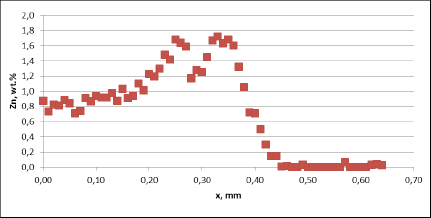
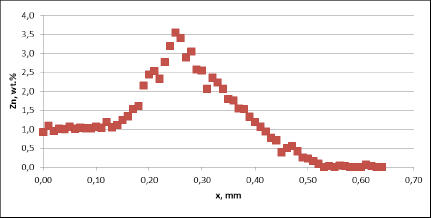
The higher corrosion rate for samples brazed with standard NOCOLOK® Flux observed in the microscopic structures has been confirmed by measuring the tube wall thickness. Results of those measurements (at least 10 measurements per point) are presented in fig. 8. It should be worth noticing that the standard variation for the wall thickness measured after 60 days of SWAAT was 10.9µm for fluxing with NOCOLOK® Li Flux and 81.2µm for fluxing with standard NOCOLOK® Flux. Such a large variation in the wall thickness in case of NOCOLOK® Flux would indicate that the “long life” capacity of the protection mechanism in this case was practically exhausted.
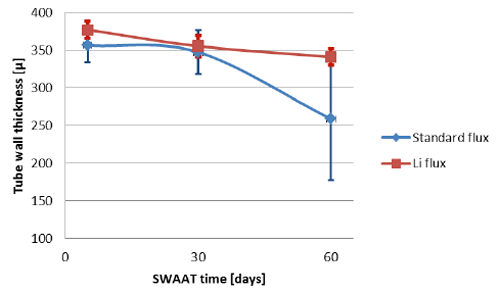
Fig. 8: Thinning of the tube wall during SWAAT
4 Conclusions
The experiment with the mock ups allowed for conclusions that there is no negative influence of NOCOLOK® Li Flux on the creation of Zn enriched sacrificial layer. Presence of NOCOLOK® Li Flux in the mixture allowed for better protection of the sample parts against corrosion in stagnant water test. One objective of stagnant water corrosion test is the simulation of stationary air conditioning unit humidity exposure.
It is possible to utilize in a flux mixture advantages of NOCOLOK® Zn and of NOCOLOK® Li. Also as shown by testing with two different tube alloys, there is no significant difference in the tube thinning between different alloys.
Initial laboratory observations – that NOCOLOK® Li Flux improves resistance to corrosion in SWAAT condition – have been proven on industrial part. Thus one option to increase the life time during SWAAT can be to use NOCOLOK® Li Flux.
Literature
[1] A. Gray, H.W. Swidersky, L. Orman.
Reactive Zn Flux – an opportunity for controlled Zn diffusion and improved corrosion resistance. AFC Holcroft 11thInternational Invitational Brazing Seminar, October 2006
[2] A. Gray, A. Afseth, H.W. Swidersky. The influence of residual flux level on the corrosion behavior of heat exchanger materials, ASST, May 2003
[3] Solvay Patent. WO10060869 A1 – Anti-Corrosive Flux – NOCOLOK®Li Flux, 2010
[4] Solvay Patent. WO11098120 A1 – Flux Forming an Insoluble Brazing Residue – Li Flux – Li3AlF6, 2011
[5] NOCOLOK®Li Flux New Brazing Flux with Improved Residue Performance