The general appearance of NOCOLOK® brazed parts can range from relatively bright to light grey depending on the flux loading and furnace dew-point. When either is increased excessively over recommended levels, the appearance moves towards the grey colour. The flux residue usually can not be seen by the naked eye, however, it is visible under a microscope at 50x magnification. Higher magnification SEM views of the flux residue are shown in the pictures below.
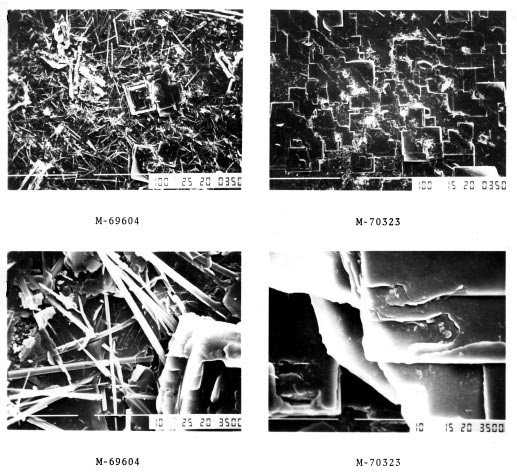
SEM photomicrographs of NOCOLOK flux residue
The pictures on the left are typical of a tunnel furnace brazed surface, needle-like in structure possibly including the odd flat platelet. The pictures identified M-70323 are typical of a furnace atmosphere containing higher than recommended levels of O2 specifically during the cooling cycle in a batch furnace. The morphology is almost 100% flat platelets. This surface has been reported to have better corrosion resistance in service.
Other flux residue properties are as follows:
a) Residue Thickness
Typically 1–2 microns. This can vary depending on flux coating weight prior to brazing.
b) Hardness
The residue hardness is about 4 on the Mohs scale.
c) Adhesion
No measurable loss has been found in circulation tests with freon or glycol type coolants using recommended flux loading. However there are reports that some detachment may occur where higher than recommended flux loading is used, particularly where molten flux pooled in downside areas.
d) Wettability
The post-braze flux residue has a hydrophilic (wetting) surface, however that wettability decreases with time.
e) Corrosion Resistance
The presence of flux residue on the part surface mildly increases corrosion resistance under normal conditions.
f) Solubility
Solubility of flux residue is influenced by the method of measurement. A typical value are between 1.2 and 3.0 g/l with Al, F and K ion concentrations approaching the stoichiometry of the compound KAlF4 .
g) Post-Braze Odour
There is a slight odour from minute amounts of H2S immediately after brazing. It disappears within a short time. If objectionable, the odour may be eliminated by rinsing the part with water.
h) Post-Treatment
The flux residue provides a good base for coatings. However, thicker residues resulting from higher than recommended flux loading can result in the poor or non-adhesion of wet or dry powder paint coatings.
NOCOLOK® Flux residue is not easily removed from the surface of brazed parts. Mechanical abrasion, such as wire brushing or grit blasting, can be used to clean off heavier flux residues from „robust“ joints. No practical chemical cleaning solution has been found. Boric acid and nitric acid solutions at higher temperature will partually remove the residues, however the times required (~ 1 hour) and the dangerous fuming with nitric acid preclude their use. Basically, the best procedure is to flux the product properly so that there is no visible after-braze residues and therefore no flux removal required.
