Technical Information by Leszek Orman, Hans-Walter Swidersky and Daniel Lauzon
Abstract
For just as long as aluminium has been used for brazing heat exchangers, there has been a trend to down-gauging components for weight savings. The most common alloying element to achieve higher strength alloys for the purpose of down-gauging is magnesium. While magnesium additions are helpful in achieving stronger alloys, the consequence is a decrease in brazeability. This article discusses the mechanism of brazing deterioration with the addition of magnesium and proposes the use of caesium compounds as a way of combating these effects.
We split the article in five parts:
- Introduction
- Effects of Mg on the Brazing Process
- Mechanism of Magnesium Interaction with the Brazing Process
- Caesium Fluoroaluminates
- NOCOLOK® Cs Flux
NOCOLOK® Cs Flux
As a more practical means of obtaining better brazeability of Mg containing alloys, a mixture of standard NOCOLOK® Flux and caesium fluoroaluminates is used. The positive influence of Cs on brazing magnesium containing alloys was previously reported in a patent for a product where potassium fluoroaluminates were mixed with caesium fluoroaluminates [11]. However, this patent covered a rather wide ratio of potassium fluoroaluminates to caesium fluoroaluminates.
The influence of actual elemental Cs content on brazeability was investigated by Garcia et al [12]. Brazeability was determined by the length of the joint obtained in a system with a gradual increase in gap clearance (similar in concept to the one shown in Fig. 1). In their work they used 6063 alloy with a Mg content of 0.66 wt%. Their major finding is presented in Fig. 6.
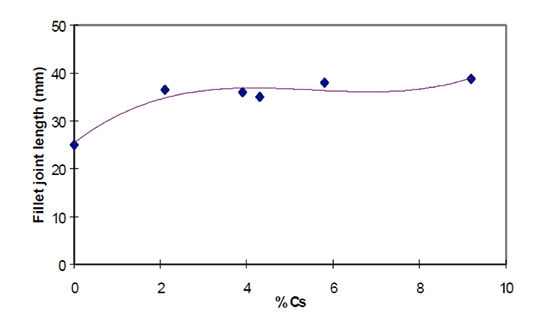
Fig. 6: Brazeability of AA6063 alloy as a function of caesium content at flux load of 5 g/m2 [12].
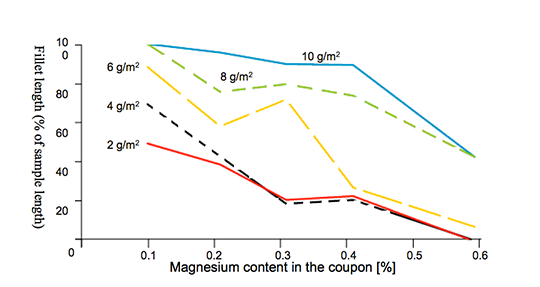
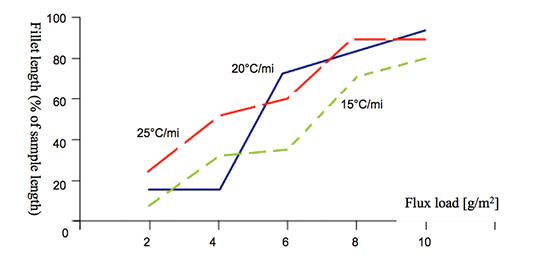
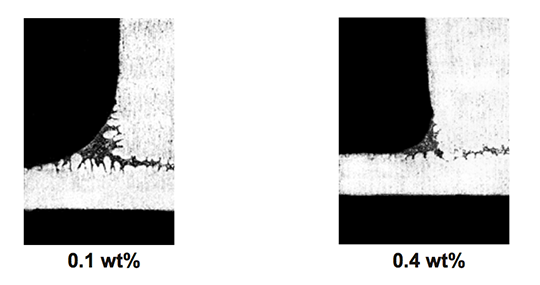
As seen in Fig. 5, even a relatively low concentration of Cs in the flux mixture improves brazeability of an alloy containing 0.66 wt% Mg. An increase of Cs concentration above 2 wt% does not lead to further improvement in brazeability. In his work Garcia et al also confirmed that faster heating rates, though positive do not significantly influence brazeability.
This work led to another important finding. By brazing small sample radiators in an industrial type furnace, Garcia et al established a practical threshold for Mg content. The flux containing 2 wt% Cs is effective for brazing aluminium alloys with 0.35% to 0.5 % Mg. At lower levels of magnesium no difference between the standard flux and the 2 wt% Cs flux was observed. Brazing samples containing 0.66% of magnesium yielded leak free parts – but the brazing ratio for fins was not fully satisfactory.
This work led to the standardization of Solvay’s NOCOLOK® Cs Flux at 2 wt% Cs. By using this minimal but effective Cs concentration in the mixture, the chemical and physical characteristics are similar to the standard flux.
Summary
- Magnesium is very often added to aluminium alloys to increase strength and machinability.
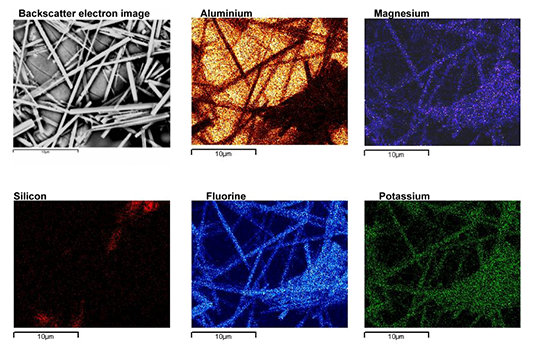
- The addition of magnesium negatively influences the brazing process due to the formation of smaller fillets and the presence of porosity in the joints. This is due to (a) magnesium diffusing to the surface during the brazing cycle and forming Mg containing oxides which are more difficult to remove by the molten flux and (b) by poisoning the action of flux through the formation of K-Mg-F compounds.
- The above effect can be made less pronounced when standard NOCOLOK® Flux is mixed with a caesium aluminium fluoride complex. At a concentration of 2 wt% Cs one can observe a positive effect on aluminium alloys containing magnesium. Increasing the Cs content above 2 wt% does not yield any further increase in brazeability.
- NOCOLOK® Cs Flux works effectively for alloys containing roughly 0.3 to 0.5 wt% Mg. Depending on specific design and process conditions, Cs containing fluxes can also offer benefits for alloys containing 0.3 wt% or even less Mg. For concentrations higher than 0.5 wt% of Mg, the effectiveness of Cs compounds in non-corrosive fluxes gradually decreases.
- Pure caesium aluminium fluoride complex is effectively used for flame brazing where a lower melting point flux is required.
Download the complete article as a PDF-File.
References:
- S. W. Haller, “A new Generation of Heat Exchanger Materials and Products”, 6th International Congress “Aluminum Brazing” Düsseldorf, Germany 2010
- R. Woods, “CAB Brazing Metallurgy”, 12th Annual International Invitational Aluminum Brazing Seminar, AFC Holcroft, NOVI, Michigan U.S.A. 2007
- T. Stenqvist, K. Lewin, R. Woods “A New Heat-treatable Fin Alloy for Use with Cs-bearing CAB flux” 7th Annual International Invitational Aluminum Brazing Seminar, AFC Holcroft, NOVI, Michigan U.S.A. 2002
- R. K. Bolingbroke, A. Gray, D. Lauzon, “Optimisation of Nocolok Brazing Conditions for Higher Strength Brazing Sheet”, SAE Technical Paper 971861, 1997
- M. Yamaguchi, H. Kawase and H. Koyama, ‘‘Brazeability of Al-Mg Alloys in Non Corrosive Flux Brazing’’, Furukawa review, No. 12, p. 139 – 144 (1993).
- A. Gray, A. Afseth, 2nd International Congress Aluminium Brazing, Düsseldorf, 2002
- H. Johansson, T. Stenqvist, H. Swidersky “Controlled Atmosphere Brazing of Heat Treatable Alloys with Cs Flux” VTMS6, Conference Proceedings, 2002
- U. Seseke-Koyro ‘‘New Developments in Non-corrosive Fluxes for Innovative Brazing’’, First International Congress Aluminium Brazing, Düsseldorf, Germany, 2000
- K. Suzuki, F. Miura, F. Shimizu; United States Patent; Patent Number: 4,689,092; Date of Patent: Aug. 25, 1987
- L. Orman, “Basic Metallurgy for Aluminum Brazing”, Materials for EABS & Solvay Fluor GmbH 11th Technical Training Seminar – The Theory and Practice of the Furnace and Flame Brazing of Aluminium, Hannover, 2012
- K. Suzuki, F. Miura, F. Shimizu; United States Patent; Patent Number: 4,670,067; Date of Patent: Jun. 2, 1987
- J. Garcia, C. Massoulier, and P. Faille, „Brazeability of Aluminum Alloys Containing Magnesium by CAB Process Using Cesium Flux,“ SAE Technical Paper 2001-01-1763, 2001