Project Study of Gel Formation in Engine Coolants – and Considerations for the Development of Further Improved Coolant Compositions Part 2
written by Dr. Alexander Rehmer (Solvay), Dr. Andreas Haas (Arteco NV), Dr. Jurgen de Kimpe (Arteco NV), Dr. Serge Lievens (Arteco NV) and Dr. Hans-W. Swidersky (Solvay).
During recent years, gel blockage in engine coolant systems with aluminum heat exchangers produced by CAB (controlled atmosphere brazing – using non-corrosive flux) has gotten more and more attention in the automotive industry. A general understanding of gel formation processes in engine coolants and the role that flux residues on internal surfaces of brazed heat exchangers may or may not have is of significant interest.
3. Comparison of gel samples collected from field return with in-laboratory generated gel samples
In this project study, the analytical investigation of two field heat exchangers comprising gel was the first important step in order to understand the gel formation in engine coolants. One gel sample was collected after a short service life while the other gel sample was obtained after a long service life. Due to evaporation of the liquid in both gel samples after a certain time, the samples were thought to be completely dry prior to characterization. However, residual ethylene glycol and water could still be detected by infrared spectroscopy (IR) in both samples.
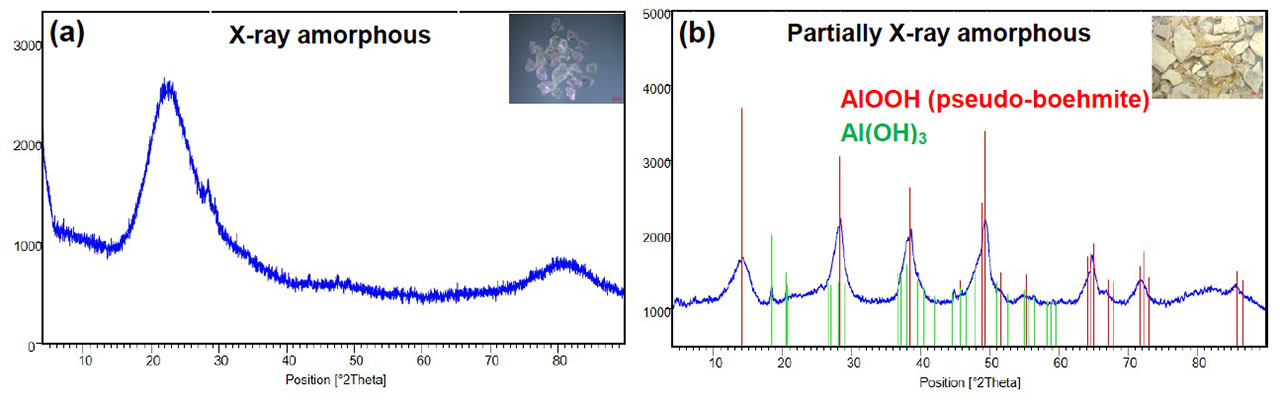
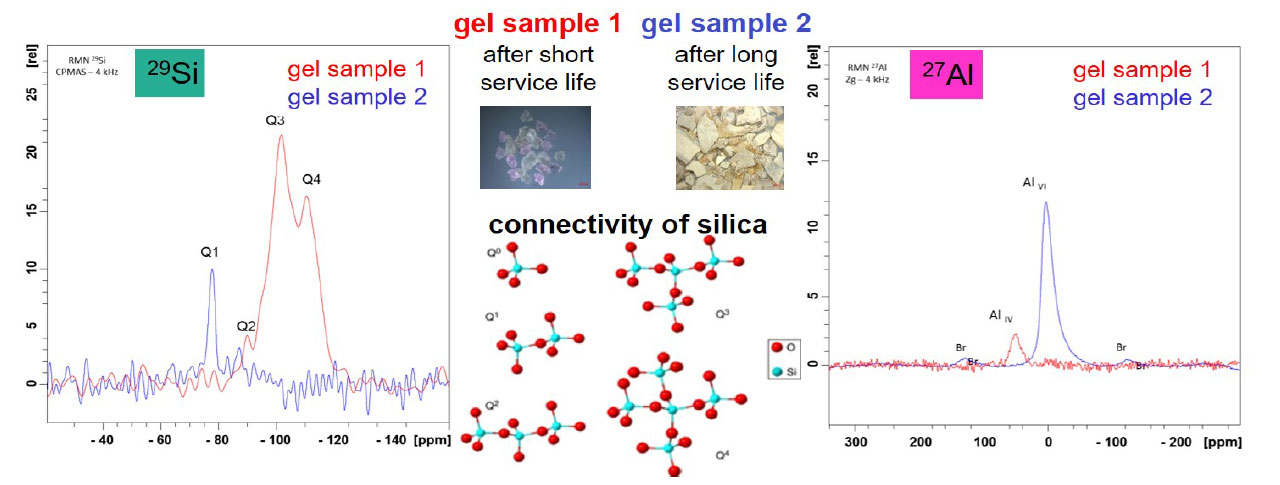
The structure of both gel samples was analyzed by X-ray diffractometry (XRD). The corresponding diffractograms of both gel samples are shown in figure 2. The samples show a different phase composition. The gel sample after a short service life is X-ray amorphous and a characterization by XRD of chemical compounds is not possible. The second gel sample after a long service life has broad reflections corresponding to AlOOH (pseudo-boehmite) which is in line with the literature.[9] Small reflections of Al(OH)₃ are detected as well. Based on these first results, further characterization of the composition of both samples was done by silicon and aluminum solid-state nuclear magnetic resonance spectroscopy (ssNMR). The gel samples were rotated during the measurement using the magic-angle-spinning technique (MAS) to improve the signal quality. In figure 3, the corresponding 27Al and 29Si MAS NMR spectra of both gel samples are illustrated. In the case of the gel sample after short service life (sample 1) the presence of silica and alumino silicates can be clearly assigned by chemical shifts of 27Al and 29Si NMR peaks in the spectra. Three 29Si NMR peaks in the range of –90 to –110 ppm correlate to Q2, Q3 and Q4 silicon sites and one 27Al NMR peak at around –50 ppm correlates to four coordinated aluminum species.[10] The NMR signals of the gel sample after long service life (sample 2) reveal a completely different phase composition. In the 29Si NMR spectrum, only one peak at –78 ppm, which corresponds to silicate dimer, is detected.[11] According to the 27Al NMR spectrum, one peak at 0 ppm could be located. The chemical shift of 0 ppm can be assigned to a six-coordinated aluminum species such as Al(OH)₃[12] or AlOOH[13].
Figure 2. XRD diffractograms of gel samples collected from field return after a short service life (a) and a long service life (b).
Figure 3. 27Al and 29Si MAS solid-state NMR spectra of gel samples collected from field return after a short service life (gel sample 1) and long service life (gel sample 2).
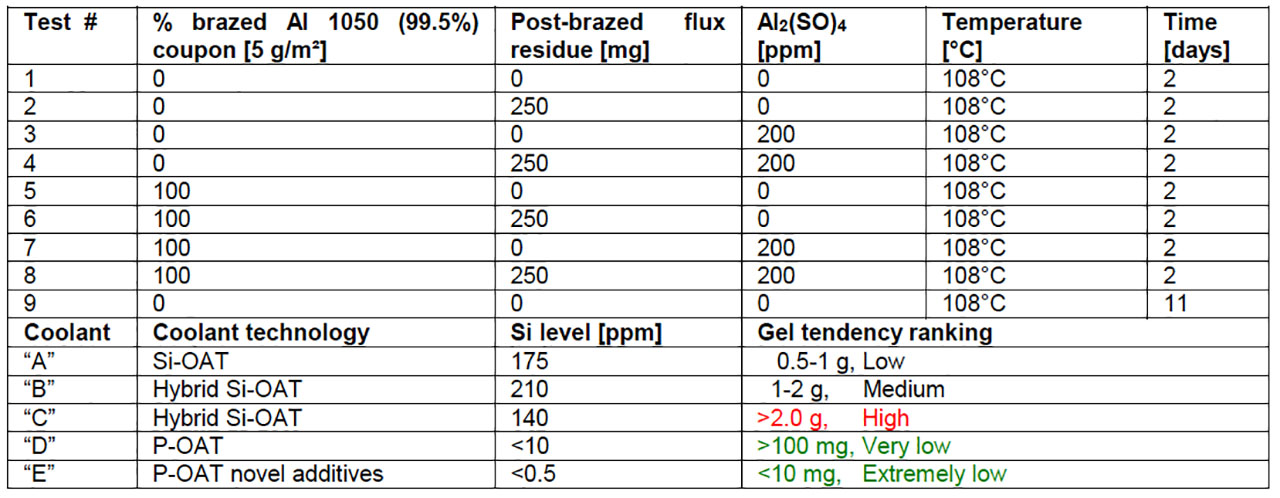
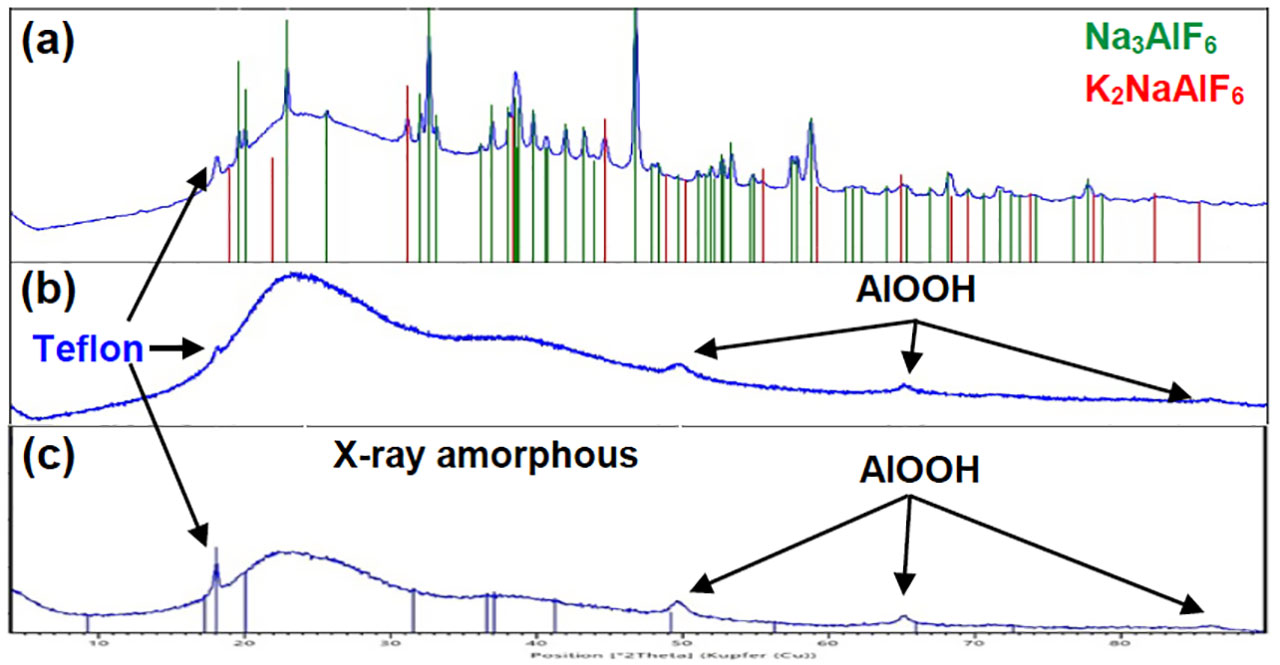
Based on the outcome of the characterization of both gel samples collected from field return heat exchangers, the simulation under laboratory conditions of such gel depositions with and without flux residue in engine coolants have been performed. The choice of appropriate parameters such as temperature, time, engine coolant inhibitor technology (Si-OAT vs. P-OAT) and the presence of aluminum species (Al material, flux residue, aluminum sulphate) is crucial to reproduce the same gel composition as found in both field return gel samples. In table 1, the complete test matrix of gel simulation under laboratory conditions is provided. It should be noted that four diluted engine coolants (glycol/water ratio: 50/50) with different corrosion inhibitor technology have been used for these trials. The outcome of the gel simulation reveals that coolant “C” has the highest and coolant “D” the lowest gel tendency of all four tested coolants, in the considered test conditions. However, the most interesting fact is that gel could be obtained without the presence of flux residue. Apparently, the presence of soluble Al³⁺ ions, which can originate in our test matrix, from either aluminum sulphate (Al₂(SO₄)₃) or by partial dissolution of the Al 1050 material during the test period, is a critical parameter in terms of gel formation. In figure 4, three selected gel samples from coolant “C” have been analyzed by XRD. The phase composition of gel samples produced with and without flux residue is different. Amorphous and crystalline components have been detected in the case of gel samples where flux residue was added to the engine coolant (Figure 4a). The crystalline compounds could be assigned as Na₃AlF₆ (cryolite) and K₂NaAlF₆ (elapsolite) which can be explained by the interaction of flux residue with sodium containing engine coolant compounds. The gel sample without flux residue and the gel sample where aluminum sulphate was added to engine coolant “C” (Figure 4b-c) are mostly X-ray amorphous. However, small reflections of AlOOH in the diffractogram could be identified. It should be noted that gel deposition in samples without flux residue (Figure 4c) were obtained after an 11 day test period. The presence of Teflon in all three gel samples can be explained by mechanical abrasion of the magnetic stir bar with the Al 1050 coupon during the test period. The composition of the in-laboratory generated gel samples without the presence of flux residue has more similarities to the real gel samples collected from field return with short operational use, compared to the samples generated in presence of flux residue. Hence, flux residue plays only a minor role in the gel formation process of our experimental set up.
Table 1: Test matrix and outcome of artificially produced gel samples in diluted engine coolant-water mixtures (Vtotal = 250 mL).
Figure 4: XRD diffractograms of artificially reproduced gel samples which have been received by using engine coolant “C” mixture with the presence of non-brazed Al 1050 coupon + post brazed flux residue (a), non-brazed Al 1050 coupon + Al₂(SO₄)₃ (b) and non-brazed Al 1050 coupon (c) after 2 days (a,b) and 11 days (c) at 108°C.
4. Conclusion
The characterization of two gel samples collected from field return heat exchangers reveals two types of gel compositions. The gel sample, which was observed after a short service life, is correlated to an alumino silicate compound. The second gel sample, collected after a long service life, consists of aluminum hydroxide oxide. According to S. Sasahara and S. Ozeki, the mechanism of gel formation to form either alumino silicates or aluminum hydroxide oxide is depending on presence of silicates, time and the amount of soluble aluminum ions in the system.
However, the role of silicate and flux residue in the gel formation process could be successfully demonstrated in the laboratory. To the best of our knowledge, no in-laboratory generated gel samples with similar chemical composition to gel samples from field service parts have been reported so far. The gel formation with and without flux residue could be observed. From this point of view, the main cause for gel formation is the presence of silicate and their stability, which is affected by the presence of Al³⁺ ions. Hence, it can be assumed that the driving force for gel formation is the reaction kinetics of the dissolution of aluminum and the generation of Al³⁺ ions.
Literature
[9] P. de Souza Santos et al., “Hydrothermal Synthesis of Well-Crystallised Boehmite Crystals of Various Shapes”, Materials Research, 2009, 12, 437-445.
[10] D.L. Gallup, “Aluminum Silicate Scale Formation and Inhibition: Scale Characterization and Laboratory Experiments”, Geothermics, 1997, 26, 483-499.
[11] O.V. Filonenko et al.,“Quantumchemical Calculation of 29Si NMR Spectrum of Silicon Dioxide Fullerene-like Molecules”, Physics and Technology of Surface, 2015, 6, 263-268.
[12] S. Ashbrook, “27Al Multiple-Quantum MAS NMR of mechanically Treated Bayerite (α-Al(OH)₃ and Silica Mixtures”, Solid State Nuclear Magnetic Resonance, 2001, 20, 87-99.
[13] P. Paluch et al., “Application of 1H and 27Al magic angle spinning solid state NMR at 60 kHz for studies of Au and Au-Ni catalysts supported on boehmite/alumina”, Solid State Nuclear Magnetic Resonance, 2017, 84, 111-117.


Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!