Project Study of Gel Formation in Engine Coolants – and Considerations for the Development of Further Improved Coolant Compositions Part 1
written by Dr. Alexander Rehmer (Solvay), Dr. Andreas Haas (Arteco NV), Dr. Jurgen de Kimpe (Arteco NV), Dr. Serge Lievens (Arteco NV) and Dr. Hans-W. Swidersky (Solvay).
During recent years, gel blockage in engine coolant systems with aluminum heat exchangers produced by CAB (controlled atmosphere brazing – using non-corrosive flux) has gotten more and more attention in the automotive industry. A general understanding of gel formation processes in engine coolants and the role that flux residues on internal surfaces of brazed heat exchangers may or may not have is of significant interest.
1. Introduction
The phenomenon of gel deposition in water-based automotive cooling systems such as radiators, heater cores, oil condensers, charge air coolers and oil coolers is well known in the industry. In the past, the stability of engine cool-ants was a challenge due to the high silicate level of the corrosion inhibitor. Silicates are one of the best-known protection against aluminum corrosion as they form a thin protective layer on the metal surfaces of the engine coolant system. Most of the commercially feasible soluble silicates in engine coolants are sodium or potassium silicates. However, the stability of such silicates under certain circumstances can deteriorate. Different key contributors can affect the stability of silicates in solution. These include high Si concentration (1400 ppm)[1], pH value below 10, presence of acidic compounds, high temperature and reaction with polyvalent ions such as Ca²⁺, Mg²⁺ and Al³⁺ to form insoluble species of silicates.[2]
Today, the instability of such silicate corrosion inhibitors is mitigated by adding appropriate stabilizers into the engine coolant. Common stabilizers can be either silicon-based molecules such as substituted organosilicon carboxylates or other derivatives such as polymers of acrylic acid with alkali metal or alkaline earth metals salts thereof.[3] A variety of silicate stabilizers exist with similar or different compositions which are used in today’s commercially available engine coolants.
Despite the addition of silicate stabilizers, the risk for gel formation cannot be totally ruled out as long as silicates will be used as a corrosion inhibitor in the engine coolant formulation. Improvements of engine coolant formulations are necessary to reduce further any kind of gel deposition. In this paper, the role of flux residue and the use of sili-cate based engine coolants with respect to the gel formation process will be presented. Furthermore, a plausible mechanism of gel formation in engine coolants will be disclosed.
2. Literature description of gel formation
The IUPAC defines the term ‘gel’ as a “Nonfluid colloidal network or polymer network that is expanded throughout its whole volume by a fluid”.[4, 5] In other words, gel can be described as a solid three-dimensional network with a specific porosity. This porosity can be filled with a liquid (wet gel) or with a gas (xerogel or dried gel). A well-known gel is silica gel (SiO₂). The general gel mechanism in case of silica gel can be explained by a two-step process, which contains the hydrolysis of silicate (Na₂SiO₃) to silicic acid Si(OH)₄ and the polymerization of polysilicic acid [SiOₓ(OH)₄₋₂ₓ]n with the formation of siloxane bonds (-Si-O-Si-). Parameters affecting gel formation are pH, temperature, time and concentration. Due to the fact that aluminum heat exchangers are widely used in the automotive field, the following section in this paper focuses on gel formation of aluminum in solution and the link to silicate stability. When aluminum is dissolved in water under acidic or alkaline conditions, the formation of aluminum hydroxide gel is initiated. Two different types of aluminum hydroxide gels can form, depending on pH of the environment. In figure 1, the two different types of gel are shown. On the one hand, formation of pseudo-boehmite (AlOOH) will take place in alkaline conditions, which can crystallize rapidly to bayerite (α-Al(OH)₃). On the other hand, the formation of pre–gibbsite will occur in acidic conditions, which crystallizes slowly to gibbsite (γ-Al(OH)₃.[6] It should be noted that small changes in a medium with alkaline pH has a significant effect on the ratio of bayerite to boehmite at the beginning of the precipitation process. Hence, small changes in alkaline pH can cause a significant difference in the phase composition of the precipitates.[7] Furthermore, the presence of Al³⁺ ions in aqueous silicate solution will promote gel formation. S. Sasahara and S. Ozeki[8] have investigated the effect of introduction of Al³⁺ ions to silicate solution. The formation of four- and six-coordinated aluminum sites (AlIV, AlVI) could be detected as a function of time. In the early stages of silicate reaction with Al³⁺ ions a four-coordinated aluminum complex forms. These four-coordinated Al complexes can be assigned towards alumino silicates (Al₂O₃-SiO₂, Al₂Si₂O₇). During subsequent reaction, the formation of six-coordinated Al complexes such as aluminum (hydroxide) oxides (Al₂O₃, Al(OH)₃, AlOOH) increases over time.
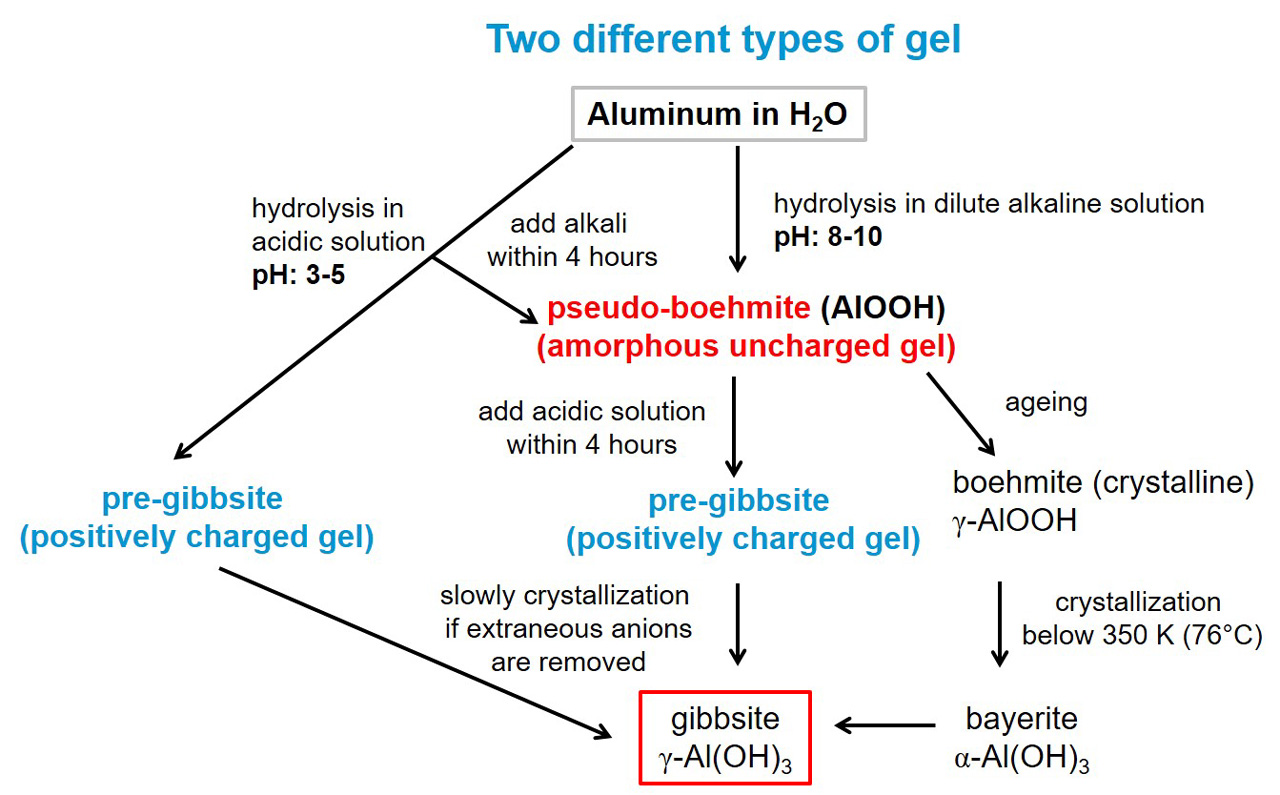
Figure 1. Simplified schematic overview of the gel formation process of aluminum hydroxide under alkaline and acidic conditions in water.
Literature
[1] Patent: Richard P. Carr, Nalco Chemical Company, “Coolant Stabilizer”, US 4707286, 1987.
[2] PQ Europe brochure, 2004, “Sodium and Potassium Silicates –Versatile compounds for your applications”.
[3] Patent: Joe C. Wilson, BASF Wyandotte Corporation, “Gelation Stabilized Water-soluble Silicates”, US 4487712, 1984.
[4] R. G. Jones; et al. 2008, T. Kitayama; W. V. Metanomski, 2008. IUPAC “Compendium of Polymer Terminology and Nomenclature”, IUPAC Recommendations 2008 (the “Purple Book”)
[5] S. Slomkowski et al., 2011. “Terminology of polymers and polymerization processes in dispersed systems” (IU-PAC Recommendations, 2011).
[6] W.J. McHardy, A.P. Thomson,”Conditions for the formations of bayerite and gibbsite”, Mineralogical Magazine, 1971, 38, 358-368.
[7] S. Musić et al., “Microstructural properties of boehmite formed under hydrothermal conditions”, Materials Science and Engineering B52, 1998, 145-153.
[8] S. Sasahara, S. Ozeki, “Effects of Al³⁺ Ions on Formation of Silica Framework and Surface Active Sites for SO₄²⁻ Ions”, Langmuir, 2016, 32, 7079-7085.

Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!