Flux Transformations
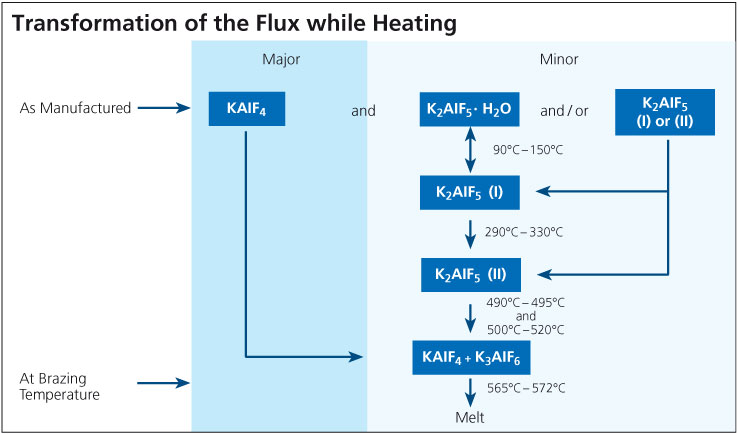
As manufactured, a non-corrosvie K-Al-F-type flux typically is a mixture of potassium tetra-fluoroaluminate (KAlF4), and also contains potassium penta-fluoroaluminate (K2AlF5). K2AlF5 exists in different modifications: potassium penta-fluoroaluminate hydrate (K2AlF5 · H2O), and hydrate-free (K2AlF5).
During the brazing process, the material undergoes essential physico-chemical alterations. While the chief component, KAlF4, is simply heated up, the compound K2AlF5 · H2O begins to lose its crystal water from 90°C (195°F) on. When the temperature is further increased within the ranges of 90° – 150°C (195°F – 302°F), and 290°C – 330°C (554°F – 626°F), two different crystallographic (structural) modifications of K2AlF5 are formatted.
When the furnace temperature is raised above 490°C (914°F), K2AlF5 begins to react chemically. According to the equation:
2 K2AlF5 → KAlF4 + K3AlF6 (Equation 1)
the exact amount of potassium hexa-fluoroaluminate (K3AlF6) necessary for a eutectic flux composition (i.e. mixture of two or more substances which has the lowest melting point; see phase diagram) is obtained from the original K2AlF5 content. At brazing temperature, the resulting flux composition has a clearly defined melting range of 565°C to 572°C (1049°F – 1062°F). The flux melts to a colorless liquid.
Due to a vapor pressure of 0.06 mbar at 600°C, some of the KAlF4 evaporates during the brazing cycle, particularly once melting temperature is reached. The total content of KAlF4 contained in the exhaust is depending on time and temperature. Based on results from TGA analysis (with a heating rate of 20°C/min), the quantity of volatile compounds in Flux between 250°C and 550°C (482°F and 1022°F) is approximately 0.2 to 0.5%. These flux fumes contain fluorides and have the potential to react with the furnace atmosphere, especially moisture, to form hydrogen fluoride according to the equation:
3 KAlF4 + 3 H2O → K3AlF6 + Al2O3 + 6 HF (Equation 2)
This is one of the reasons, why the brazing process should take place in a controlled atmosphere (nitrogen) with low dew point and low oxygen level (another reason is to minimize re-oxidization effects on the aluminum surfaces).
Directly after brazing has been completed, flux residues consist mainly of KAlF4 and K3AlF6. In the presence of moisture from the surrounding atmosphere, the K3AlF6 is converted back to K2AlF5 · H2O over time (several days) in a reaction reverse to the one described in equation 1 followed by a re-hydration step.
The schematic below illustrates the transformations that occur as the flux is heated to brazing temperature. Note that these phases are unstable outside the furnace atmosphere.

Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!