Aluminium Flame Brazing – Basics Part 1
Flame brazing of aluminum is not new. In fact the very first brazed aluminum assemblies were produced using a chloride based flux and a flame as the heat source. What has changed over the years is the sophistication of the types of fluxes available and to a certain extent the alloy selection.
Introduction
However, even if one returns to the absolute basics of a flame, filler metal and flux, there remains a great deal to be learned about the fundamentals of flame brazing of aluminum. This becomes especially evident when the brazing engineer applies his techniques and equipment to NOCOLOK ® Flux flame brazing and years of learned practice seem to fail. This is largely due to the fact that the years of acquired knowledge of flame brazing aluminum has come from corrosive chloride-based flux brazing. Unfortunately, the same techniques can not be directly applied to NOCOLOK ® Flux flame brazing. It is therefore the intention of this article to re-familiarize the brazing engineer with the fundamentals of flame brazing aluminum and use those fundamentals to realize all the advantages of NOCOLOK ® Flux brazing.
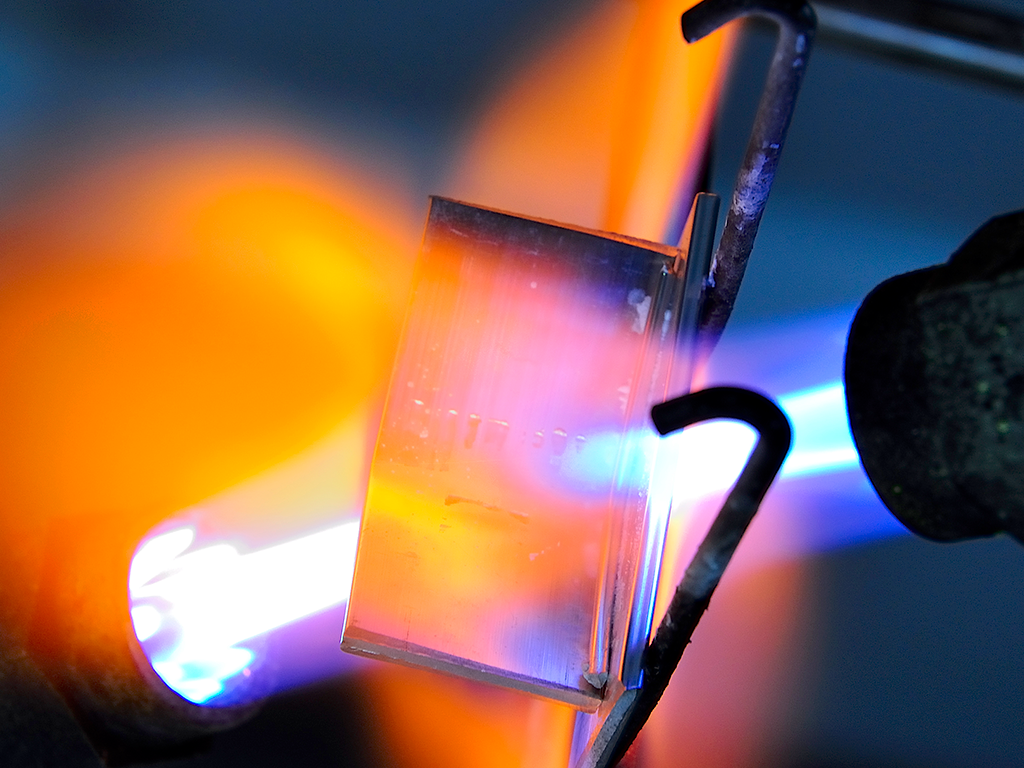
What is Flame Brazing?
According to the American Welding Society, brazing is the joining of metals using a molten filler metal, which on cooling forms a joint. The filler metal melting temperature is above 450 °C, but below the melting point of the metals.
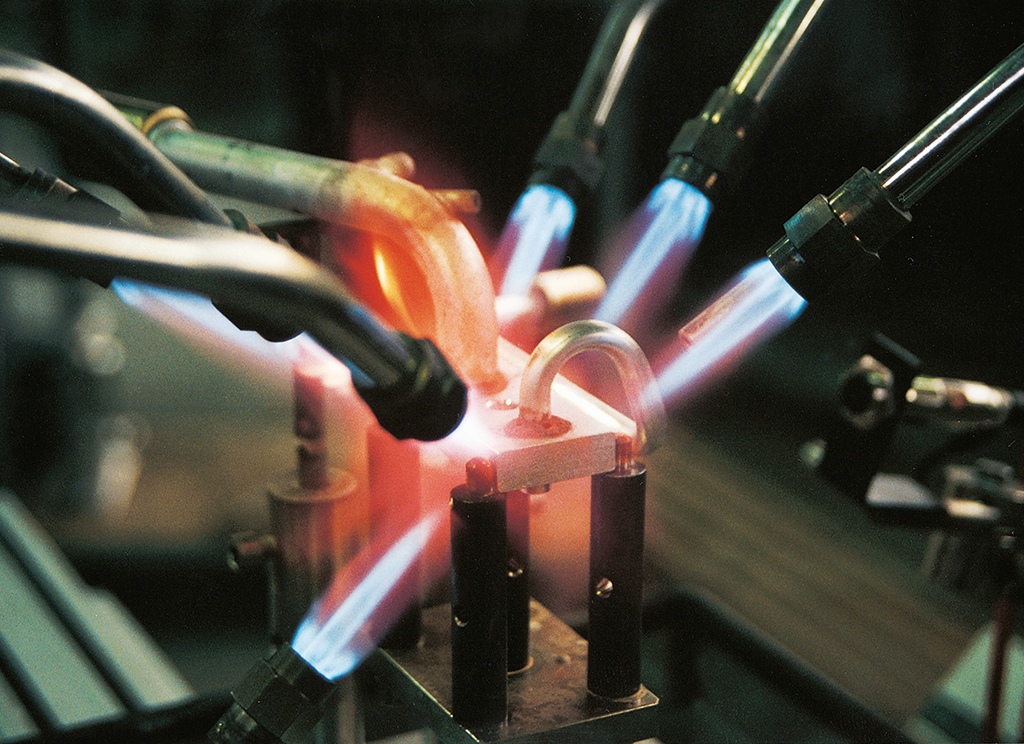
Flame brazing then implies the use of a flame as the heat source to accomplish what is described above.
Flame brazing lends itself well to joining components with simple configurations such as tube-to-tube, tube-to-fitting and joints having large thermal mass differences. Since much faster heating rates are possible than in furnace brazing, flame brazing is versatile and as will be explained in more detail later, can braze some Mg containing alloys.
What is NOCOLOK ® Flux?
NOCOLOK ® flux is a white powder consisting of a mixture of potassium fluroaluminate salts of the general formula K1-3 Al‑F4-6. The flux has a defined melting point range of 565 °C to 572 °C, below the melting point of the Al-Si brazing alloy. The flux is non-corrosive and non-hygroscopic and is only very slightly soluble in water (0.2 % to 0.4 %). The shelf and pot life of the flux is therefore indefinite. The flux does not react with Al at room temperature or at brazing temperature and only becomes reactive when molten.
Role of the Flux
Once molten the flux works by dissolving the oxide film on the Al surfaces to be joined and prevents further oxidation. The flux wets the Al surfaces and allows the filler metal to flow freely into the joints by capillary action. Upon cooling, the flux solidifies and remains on the surfaces as a thin, tightly adherent film, which need not be removed.
Joint Clearances
The recommended gap tolerances for flame brazing range from 0.1 mm to 0.15 mm. Larger gap clearances can be tolerated, but capillary action is reduced, gravity activity is increased and more filler metal may be required. Friction fits should also be avoided as this will restrict filler metal flow and result in discontinuities in the brazed joint area.

When it comes to aluminum brazing, safety is of the utmost importance. I appreciate what you had to say about joint clearances. If I were to need such a service, I would make sure to work with the best company in the business.
I can’t believe that 450 degrees Celcius is still below the melting point of the metals! Were the fillers engineered specifically to melt at high temperatures? I’ll have to do more research before I try doing any of this myself.