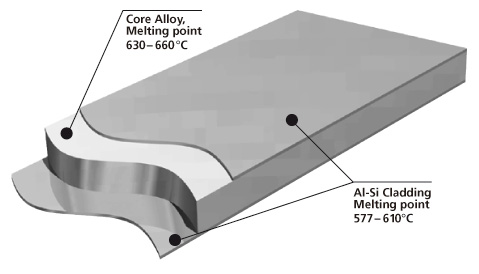
Brazing sheet comprises of a core alloy clad on 1 or 2 sides with a lower melting aluminum-silicon (Al-Si) alloy. This thin layer, usually makes up 5 % to 10 % of the total thickness of the brazing sheet.
It melts and flows during the brazing process, to provide upon cooling a metallic bond between the components. It is common that the braze clad alloy are from the AA 4xxx series or more particularly AA 4343 (Al-6.8~8.2 wt.% Si).
However, if larger fillets are desirable, or if in a situation where brazing is likely to occur at lower temperatures, AA‑4045 is the preferred choice.
Manufacturing

Cross-section morphology of a double-side clad tubestock.
Aluminium brazing sheet is manufactured by roll-bonding techniques to clad a core alloy ingot on one side or both sides with a low melting AlSi alloy. As an alternative, one side can be clad with a non-braze alloy, e.g. Zn-containing alloy.
Depending on the desired final properties, the core is either homogenized or not before the cladding operation.
The whole package is subjected to preheating, hot rolling and cold rolling down to the final thickness of the respective products. Depending on the requested final properties, the material is subjected to final annealing and / or intermediate annealing operation(s).
The core provides structural integrity. It is common to use a variety of aluminium alloys, examples being AA 3xxx (AlMn), more particularly AA 3003, AA 3005, AA 3105 and modified versions of AA 3003 or AA3005 (long life alloys).
Phase diagram
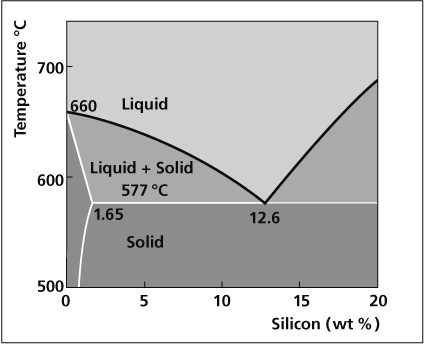
Aluminum End of Al-Si Phase Diagram
The melting characteristics of the cladding alloys are governed by the Al-Si phase diagram. The eutectic composition, i.e. the amount of Si required to produce the lowest melting point is 12.6%. The melting point at this composition is 577°C. At lower Si levels the solidus or the point at which melting begins is also 577°C. However, melting occurs in a range and the temperature above which the filler is completely molten is called the liquidus. In between the solidus and liquidus, the filler is partially molten, existing both as liquid and solid. The difference between the solidus and the liquidus forms the basis for various filler metal alloys.
The table below shows the solidus and liquidus of common brazing alloys.
[table id=2 /]
The higher Si alloys (e.g. AA4047) have higher fluidity and a narrower melting point range while the lower Si alloys have less fluidity with a wider, higher melting point range. Erosion of the base metal occurs when the braze alloy dissolves part of the core alloy. The extent of erosion is increased by:
- Higher Si levels in the braze alloy
- Longer braze cycles
- Excessive peak brazing temperatures
- Excessive thickness fo the braze metal layer
- A design which allows pooling of the braze metal to occur

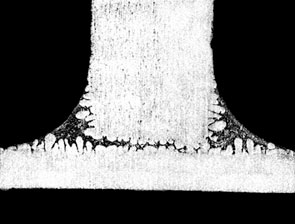
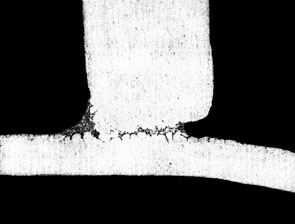

 flame brazing of aluminium to copper.
flame brazing of aluminium to copper.