Flux Characteristics and Transfer Systems in Electrostatic Application – Part 5

Flux Powder Adhesion:
Dust formation and flux fall off are of general interest to the brazing industry. Regardless of the application method, dust generation (particularly airborne fines) must be avoided or kept to a minimum. If dust formation cannot be prevented, local exhaust ventilation and meticulous housekeeping are recommended.
The inhalation of flux dust in high concentrations over a long period of time constitutes a health hazard to exposed personnel. Due to the abrasion caused by flux dust, unprotected equipment surfaces of moving parts can show premature deterioration if not regularly maintained.
As mentioned above, flux adhesion in dry application is lower than in wet application. Forced convection heating zones are one possible area in the process where flux losses may occur. Other factors might be manual transfer of units or vibrations during mechanical transport. Some users improve adhesion by applying the powder on surfaces still lubricated with residual evaporative oils.
When excess flux dust is generated in the drying oven or the furnace, it can get into the exhaust steam and create difficulties with the exhaust treatment (i.e., quickly overload the filter or scrubber). If the exhaust is treated with thermal or catalytic processes (i.e., incineration of evaporative lubricants), separation of solid and gaseous components can become necessary.
Excess flux dust in the brazing furnace can also settle on the conveyor belt or on the furnace muffle. The conveyor belt can take this powder through the brazing zones, where it eventually melts. This may contribute to accelerated corrosion.
Users of dry fluxing technology are aware of the reduced flux adhesion. At most of the operations we were allowed to visit, dust formation due to flux fall off is kept to minimum levels by appropriate technical installations.
Experiments for Flux Powder Adhesion:
We researched the ability of flux powders to adhere to aluminum surfaces in electrostatic application. A very simple test was used to determine adhesion tendencies. This experimental arrangement is not simulating real production conditions. Nevertheless, it provides very useful information.
A plain square aluminum plate (0.5 m x 0.5 m) is electrostatically coated on one side with flux powder. The total flux weight is determined to calculate flux loading. The plate is then dropped (in vertical position) from 5 cm height to the ground and the flux loss is registered as percentage of original flux weight.
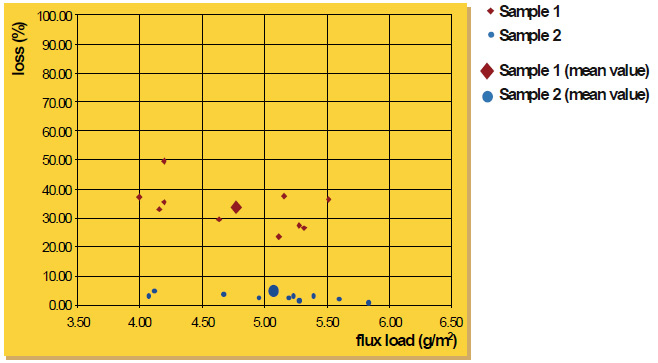
Attached is a diagram with the results for flux sample 1 and sample 2. For each material, ten measurements were performed. There is a certain variation of the individual figures; nevertheless, the trend is obvious. Sample 1 (”coarse” material) shows an average loss of approximately 33% compared to approximately 3% of sample 2 (”fine” material). This general tendency of powder with larger particle size distribution to adhere less than fine powder was also confirmed by additional experiments we made. The flux fall off in wet flux application under these test conditions is approximately 1%.
Dry Flux Application on an Aluminum Plate (0.25 m2) Flux Loss for a Fall from 5 cm Height
Flux powder is electrically charged in the gun. Usually, adhesion in electrostatic application is dependent on electrical forces. The flux loses the charge when it hits the grounded heat exchanger. Gravitational forces are now competing with relatively weak Van-der-Waals forces. This explains why fine particles adhere better.
Large flux grains are affected more by gravity, and consequently fall off more easily. We were able to synthesize a flux with very large average particle size distribution which fluidized perfectly (spray factor 143 g/0.5 min; i.e., twice as ”good” as sample 1). However, when this material was used for electrostatic application, the air flow from the spray gun blew away a lot the flux just deposited on the surface.
To be continued …

Hinterlasse einen Kommentar
An der Diskussion beteiligen?Hinterlasse uns deinen Kommentar!