Approach to non-corrosive fluxes for further reduced residue solubility and improved magnesium tolerance
Technical Information by Ulrich Seseke-Koyro, Hans-Walter Swidersky, Leszek Orman, Andreas Becker, Alfred Ottmann
We split the article in four parts:
- Abstract and Basic Experimental Laboratory Procedures
- Reduced Flux Residue Solubility
- Improved Magnesium Tolerance
- Summary and Outlook
Improved Magnesium tolerance
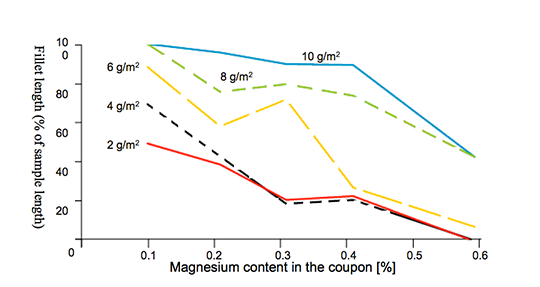
Mg additions to Al alloys contribute to higher strength properties. The ongoing trend in saving weight by down-gauging of Al sheet thickness requires sufficient mechanical stability. One option for the production of higher strength Al alloys is to increase the Mg content.
A disadvantage of Mg is the interaction with potas-sium fluoroamuminate fluxes during brazing, which results in poor joint formation [3] [4]. This effect, known as “flux poisoning”, is caused by the formation of high melting compounds. The addition of caesium and other metals to the flux helps to compensate to a certain degree the poisoning [6].
For the first set of laboratory brazing experiments we chose commercially available AMAG 6951 brazing sheet (0.68% Mg, 4343 clad) and clad-less AMAG angle material (0.68% Mg) to investigate the brazing performance and joint formation. In this situation the metal-to-metal interface adds up to 1.36% Mg (2 x 0.68%) in total.
Table 1 shows a list of representative flux combina-tions with NOCOLOK® types, KAlF4, Li3AlF6. CsAlF4, and AEFs.
We repeated all brazing tests with each sample three times.
| Flux Type | Load | Fillet visual validation | Comment |
|---|---|---|---|
| NOCOLOK® Cs Flux | 10 g/m2 | 100% | very small joint inconsistent seam |
| MD001212 LiCs24 | 10 g/m2 | 100% | small joint weak seam |
| MD001223 LiCs43 | 10 g/m2 | 86% | small joint inconsistent seam |
| AB039215 KAlF4/BaF2 | 10 g/m2 | 52% | small joint inconsistent seam |
| NOCOLOK® Cs Flux | 15 g/m2 | 100% | weak seam |
| MD001212 LiCs24 | 15 g/m2 | 100% | thicker than with NOC Cs Flux |
| MD001223 LiCs43 | 15 g/m2 | 100% | thicker than with NOC Cs Flux |
| AB039215 KAlF4/BaF2 | 15 g/m2 | 98% | weak seam slighly better than NOC Cs Flux |
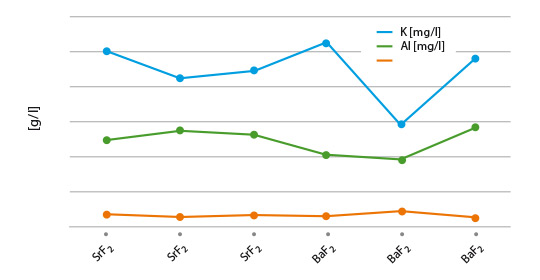
Table 1: Brazing trials: AMAG clad – AMAG clad-free angle different flux blends based on KAlF4 plus BaF2/Li3AlF6/CsAlF4
The angles from most of the AMAG specimens could be removed after brazing by pulling. Only a broken inner and outer fractured seam line was left – as can be seen below in picture 1 a.
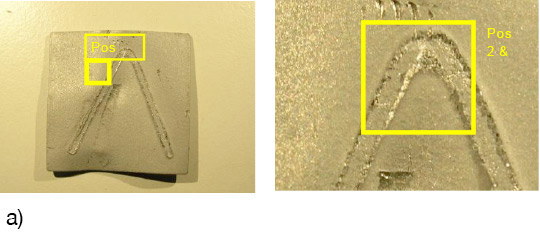
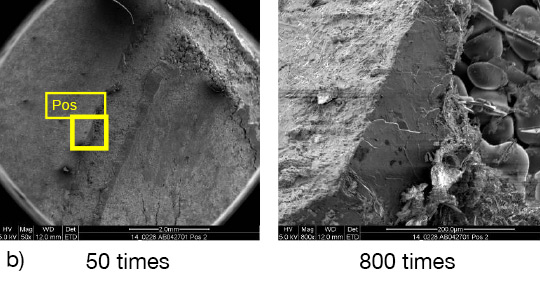
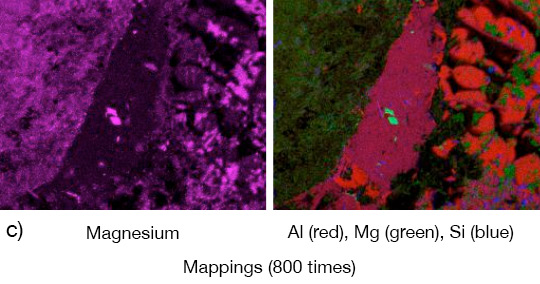
Picture 1: a) Photos, b) and c) SEM/EDX of NOCOLK® Cs Flux brazed sample (load 15 g/m²) Coupon 0.68% Mg, angle 0.68% Mg – angle removed by pulling
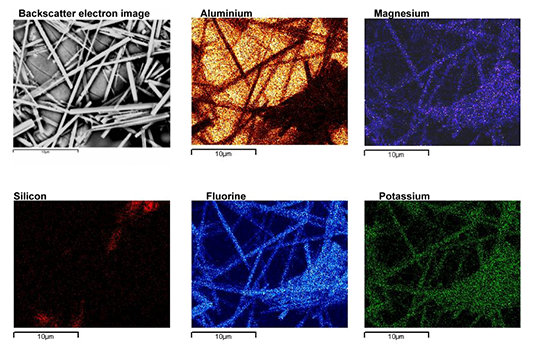
From the SEM analysis it is evident that a proper met-allurgical joint between base and angle has not been formed.
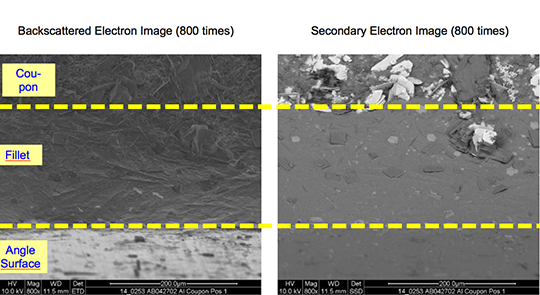
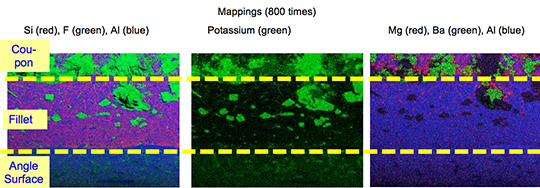
Picture 2: SEM/EDX analysis of aluminium ‘angle on coupon‘ brazed with KAlF4/BaF2 blend
There is flux residue present in the pulled apart fillet. This indicates that the liquid filler alloy was not capa-ble of pushing out completely the flux of the joint and it could be an explanation for the weakness of the fillet.
However, in case of the blend MD001212 LiCs24 with load 15g/m2 the joint structure is thorough as can be seen in picture 3 a).
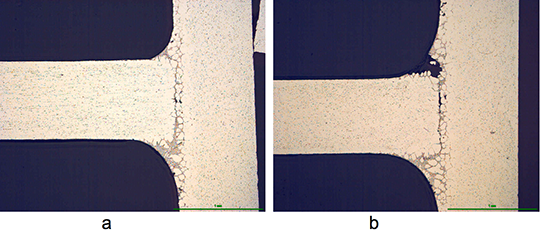
Picture 3: Microstructures of the brazed joints
a) Flux MD001212, load 15g/m2
b) Flux MD001223, load 15g/m2
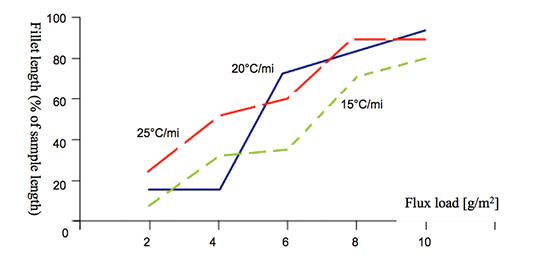
It is worth mentioning when connecting blocks are brazed to condenser manifolds often a high load of manually applied flux is used in order to overcome the high Mg content in the block material. For such a case using the mixture MD001212 would allow for having quite high Mg content in the block material, which can be required by the designers of condens-ers.
The total concentration of 1.36% Mg (joint interface) is probably too high, because for most brazing applica-tions, a flux load of 15g/m2 is impractical. Thus, we decided to reduce the level of Mg in our samples to half – i.e. to 0.68% – by switching to an AA1050 (Al 99.5%) angle. We also reduced the flux load to a more process-typical level of 5g/m². The results are listed in table 2:
| Flux Type | Load | Fillet visual validation | Comment |
|---|---|---|---|
| MD001212 LiCs24 | 5 g/m2 | 100% | good seam |
| NOCOLOK® Cs | 5 g/m2 | 87% | small joint |
Table 2: Brazing tests AMAG coupon (0.68% Mg)/Al99.5 angle
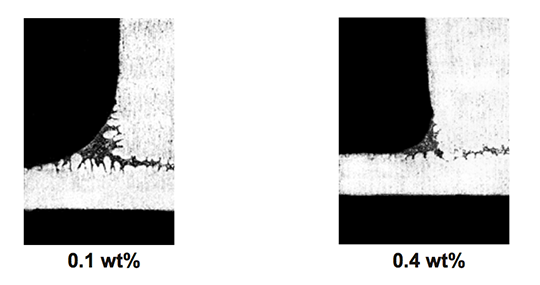
The structure of the joint cross section below (picture 4) obtained with flux MD001212 LiCs24 shows good quality.
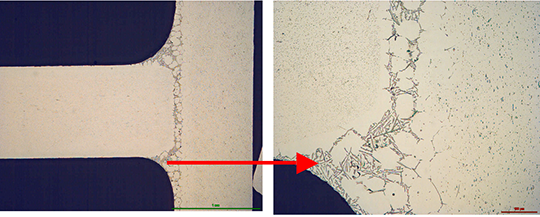
Picture 4:Joint cross sections of alloys containing 0.68% Mg brazed with MD001212 LiCs24, load 5g/m2
To be continued…
- P Garcia et al, Solubility Characteristics of Potassium Fluoroaluminate Flux and Residues, 2nd Int. Alum. Congress HVAC&R, Dusseldorf (2011)
- P Garcia et al., Solubility and Hydrolysis of Fluoroaluminates in Post-Braze Flux Residue, 13th AFC Holcroft Invitational Aluminum Brazing Seminar, Novi (2008)
- J Garcia et al, Brazeability of Aluminium Alloys Containing Magnesium by CAB Process Using Cs Flux, VTMS5, 2001-01-1763 (2001)
- H Johannson et al, Controlled Atmosphere Brazing of Heat Treatable Alloys With Cesium Flux, VTMS6 C599/03/2003 (2003)
- Handbook of Chemistry and Physics; Ref. BaSO4: 0.0025 g/l
- U Seseke, Structure and Effect – Mechanism of Flux Containing Cesium, 2nd Int. Alum. Brazing Con., Düsseldorf (2002)