1. Preparation and Application
This article provides information about the application of binder systems for NOCOLOK Flux.
Solvay offers three concepts for flux binder application:
- NOCOLOK Binder (water-soluble) / NOCOLOK Thickener (water-soluble)
- NOCOLOK System Binder (water-based)
- NOCOLOK Flux plus Binder Mixture (water-based)
These products can be used in water-based NOCOLOK Flux slurries to improve flux particle adhesion. This is of particular interest for fluxing of pre-formed components prior to assembly in order to reduce flux fall-off and dust formation. Binders are also helpful to pre-coat certain areas with specific flux loads. All binder mixtures can be applied on external and internal surfaces.
During the brazing cycle, these binders will completely evaporate (mostly between 350 and 400°C). When used as described below, there will neither be detrimental interactions between the binder and the flux, nor between the binder and the aluminum surfaces. Trials have shown that even at four-times the standard flux load with a binder mixture there was still no surface discoloration after brazing.
2. General Comments
The surface areas to be coated with binder mixtures must be free of lubricants, oils, dirt, and dust. Means of application include spraying, dipping, and brushing.
All NOCOLOK Flux binder mixtures can be applied by spraying with a suitable spray gun (1.4 mm – 1.6 mm) at approximately 3 – 5 bar pressure.
The surface temperature should be at least 10°C.
When binders are used for flux application, the recommended flux load for good brazing results is the same as it is for the standard process (i.e. between 3 – 5 g/m2). The thickness of the binder coating is usually between 10 – 30 μm.
Drying can be done in air – requiring approximately 15 – 20 minutes at room temperature for the coating surface – and 50 – 60 minutes before the parts can be handled. Oven and forced convection drying is feasible too: at 50 – 80 °C, parts will dry within 5 – 20 minutes.
Please refer to the MSDS for detailed information regarding the safe handling of the product.
3. Preparation of Binder Mixtures
For all binder concepts and preparations, the mixtures should be prepared or opened immediately before consumption.
To prepare a mixture free of agglomerates and to achieve best coating results, the following procedures must be observed for either binder concepts:
- NOCOLOK Binder / NOCOLOK Thickener
- 45 parts (wt%) de-ionized water (as used for preparing standard flux slurries) is mixed thoroughly with
- 15 parts (wt%) NOCOLOK Binder (water-soluble) and
- 5 parts (wt%) NOCOLOK Thickener (water-soluble).
- Once the first three components are completely homogenized,
- 35 parts (wt%) NOCOLOK Flux powder are added successively under continuous agitation.
- NOCOLOK System Binder
- NOCOLOK System Binder (water-based) already contains the binder and thickener component as well as water. Consequently, only NOCOLOK Flux powder must be added.
- 65 parts (wt%) NOCOLOK System Binder (water-based) plus
- 35 parts (wt%) NOCOLOK Flux.
- NOCOLOK Flux plus Binder Mixture
- NOCOLOK Flux plus Binder Mixture (water-based) is a ready-for-use preparation containing NOCOLOK Binder, NOCOLOK Thickener and NOCOLOK Flux powder.
If necessary, the mixtures can be passed through a sieve prior to use. This will remove any potential agglomerates.
Prior to use the flux powder in the mixture must be suspended. The thickener will prevent the flux powder from settling too fast, however, when stored for some time or diluted, the mixture must be well shaken before spraying.
The binder component is activated by oxygen from air. Once sprayed and dried, the product cannot be recycled or reused.
Any remaining flux / binder mixture should be stored in an airtight and sealed container. We recommend consuming the mixtures within one week after mixing.
4. Additional Information:
- NOCOLOK Binder, -Thickener, and –System Binder are compatible for standard NOCOLOK Flux, -LM Flux, -Cs Flux, and -Sil Flux. They are not suitable for NOCOLOK CB Flux and -Zn Flux due to chemical reactions between these fluxes and the ingredients.
- The formulations (mixing ratios) provided in Solvay’s technical information sheets and brochures are intended as general recommendations – They provide the best basis for automated spray application and have been tested with good brazing results.
- The recipes can be adjusted to specific application needs by changing the mixing ratios within certain ranges.
- A well balanced ratio of binder and thickener to flux in the mixtures is important for good brazing performance:
- Higher binder ratios result in a harder coating layer and stronger flux adhesion. But they require more care for the binder removal step.
- Very high binder and/ or thickener ratios increase the organic content in the mixture – which may result in carbon residues (discoloration) after brazing.
- It is possible to reduce and/ or to increase the water content of the mixtures – resulting in higher respectively lower viscosity.
- Water dilution will cause less wetting action and reduced adhesion.
- A surfactant (wetting agent) is part of the binder formulation – providing uniform coating, and – compensating (to some extent) for surfaces not cleaned prior to application.
- Thickener is used for adjusting the viscosity and to keep the flux powder longer in suspension – This provides better performance in spray application. Nevertheless, formulations can be prepared and used without the addition of thickener.
- Cleaning before binder-based flux application is recommended – but not mandatory.
- A clean surface can be coated more easily and the flux adhesion will be better.
- Residual oils and lubricants are reducing binder activity and require higher flux load.
- Higher surfactant levels can compensate for some contamination – but result in more foaming.
5. Binder Flux Mixing Ratios
- The standard composition is 35% NOCOLOK Flux, 15% NOCOLOK Binder, 5% NOCOLOK Thickener and 45% water. If a product with lower flux ratio is wanted (i.e., with only 10% flux), the composition must be modified. Right now, we are proposing 10% flux, 8% binder, 2% thickener and 80% water. There is only limited experience with this composition, and we are a little concerned. The reasons for our concerns are as follows:
- With 35% flux, the ratio of flux to binder/ thickener on the surface of the headers is sufficient to combat the effects of the high organic content. Also, 15% binder has reasonable adhesion characteristics.
- At 10% flux, the ratio of flux to binder/ thickener must be modified; otherwise there may not be sufficient flux to combat the high organic content. This is why we propose to reduce the binder and thickener to 8% and 2%, respectively. In other words, too much binder/ thickener and not enough flux may lead to black deposits on the headers after brazing and/ or difficulties in brazing.
6. Warehousing Considerations and Shelf Life
- Under standard storage conditions, the shelf life is up to 12 months and probably longer. Standard storage conditions means that the product was stored at less than 30°C, as suggested in the MSDS.
- The binder product can be stored at a temperature higher than 30°C, but the shelf life will shorten due to premature aging. Therefore, we recommend that the binder products be consumed within six months, if the storage temperature is a constant 40°C. This is not based on experimental data, but on general knowledge of water based polymer systems and adhesives. Any product stored at a temperature higher than 40°C should be consumed more quickly.
- Under no circumstances should the binder products, in their original packaging, be exposed to a temperature of 60°C or above. We suspect that polymerization will occur, agglomerates will form and the performance will drop.
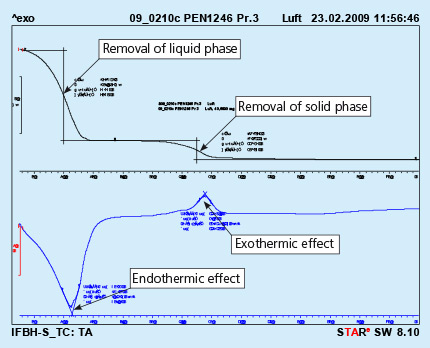
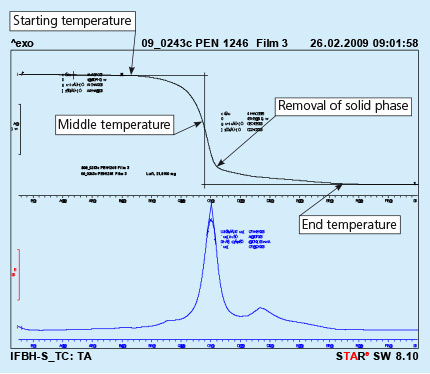
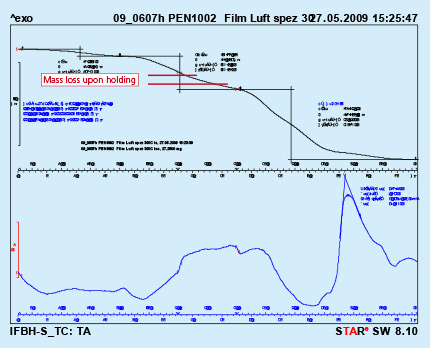
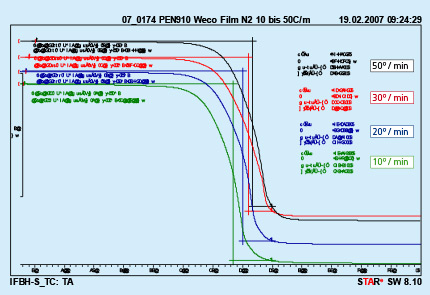
7. Thermo-Gravimetric Analysis (TGA) for Binder Flux
Please refer to the flyer “NOCOLOK Flux Application with Binders”.
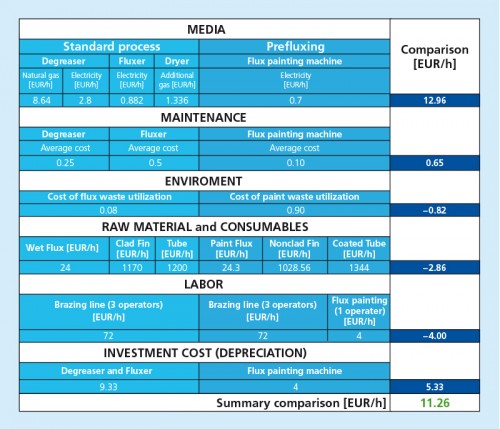
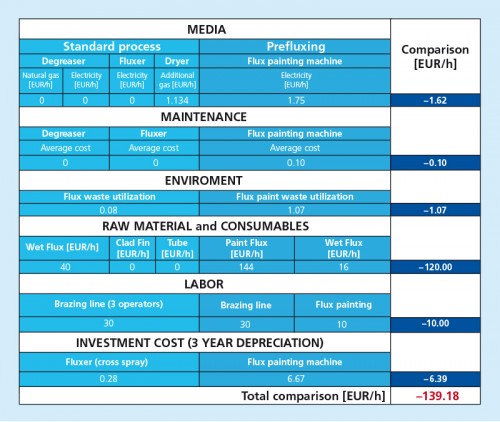
8. Recommendations for Reducing Costs
- Is not possible to only mix the binder, thickener and flux and just add the water on site. Without the water, the flux/ thickener/ binder mixture forms a rubbery-like substance that is very difficult to work with.
- The least expensive option is to purchase the binder and thickener separately and do mixing of all ingredients on site. The most convenient option is to have a ready-mix, ready-to-use product supplied.
- Please see above for additional recommendations for mixing.